21:3 Classifying Chemical Reactions
... called fungi. There are many kinds of yeasts, some of them of great importance to humans. Yeast is necessary to make leavened bread, beer, and cheese. It is rich in B vitamins; a form of yeast called brewer's yeast is used as a diet supplement. Yeasts are found in the soil, in water, on the surface ...
... called fungi. There are many kinds of yeasts, some of them of great importance to humans. Yeast is necessary to make leavened bread, beer, and cheese. It is rich in B vitamins; a form of yeast called brewer's yeast is used as a diet supplement. Yeasts are found in the soil, in water, on the surface ...
How do we predict chemical change?
... Not every combination of substances will lead to the formation of new compounds via a chemical reaction. How can we predict when a chemical process takes place? One approach could be to compare the relative stability of reactants and products. We might expect that chemical reactions will proceed in ...
... Not every combination of substances will lead to the formation of new compounds via a chemical reaction. How can we predict when a chemical process takes place? One approach could be to compare the relative stability of reactants and products. We might expect that chemical reactions will proceed in ...
Notes
... • Physical property - is observed without changing the composition or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ...
... • Physical property - is observed without changing the composition or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ...
Section B - 8 UNO NON-WASTE CHEMICAL STORAGE
... are listed below. These procedures are not intended to be all-inclusive, but should form the basis for more specific procedures in the workplace. Specific instructions on chemical storage may be obtained from the MSDS, container label, or by contacting EHS. B. General Storage The following general p ...
... are listed below. These procedures are not intended to be all-inclusive, but should form the basis for more specific procedures in the workplace. Specific instructions on chemical storage may be obtained from the MSDS, container label, or by contacting EHS. B. General Storage The following general p ...
Learning Activities
... reactant, shift left, shift right, concentration, endothermic, exothermic, catalyst, mole, avagadro’s number, one step conversion, two step conversion, hydrate, empirical formula, molecular formula, stoichiometry, mole ratio, percent yield, and theoretical yield. ...
... reactant, shift left, shift right, concentration, endothermic, exothermic, catalyst, mole, avagadro’s number, one step conversion, two step conversion, hydrate, empirical formula, molecular formula, stoichiometry, mole ratio, percent yield, and theoretical yield. ...
THE GAMMA FOREST AT BROOKHAVEN
... Data obtained in the course of a recent study (Superina 1998) ranged from sediment chemistry and size to vegetational analysis. This paper will show geobotanical relationships by integrating the geological, chemical, and botanical data obtained during the study. The data was obtained in the Gamma Fo ...
... Data obtained in the course of a recent study (Superina 1998) ranged from sediment chemistry and size to vegetational analysis. This paper will show geobotanical relationships by integrating the geological, chemical, and botanical data obtained during the study. The data was obtained in the Gamma Fo ...
Thermodynamics of Micelle Formation
... To determine the degree with which different surfactants exhibit variable thermodynamic profiles, we performed a titration of CTAB (cetyltrimethylammonium bromide) into aqueous solution. Peaks 1 through 20 are endothermic and peaks 21 to 50 are exothermic (Fig. 3A). This data was unexpected as the t ...
... To determine the degree with which different surfactants exhibit variable thermodynamic profiles, we performed a titration of CTAB (cetyltrimethylammonium bromide) into aqueous solution. Peaks 1 through 20 are endothermic and peaks 21 to 50 are exothermic (Fig. 3A). This data was unexpected as the t ...
Chemistry 20H
... 1. Identify changes which indicate that a chemical reaction has taken place. 2. Define chemical and physical reactions. 3. Understand why a chemical reaction occurs. 4. Understand that both atoms and mass are conserved in chemical reactions: the law of conservation of mass. 5. Be able to write, unde ...
... 1. Identify changes which indicate that a chemical reaction has taken place. 2. Define chemical and physical reactions. 3. Understand why a chemical reaction occurs. 4. Understand that both atoms and mass are conserved in chemical reactions: the law of conservation of mass. 5. Be able to write, unde ...
Separating Substances
... The process of purifying metals by recycling plants has been simplified for the purposes of this lab. For example, during the real process, other materials may be grouped with the aluminum and tin cans, such as plastics. Furthermore, the lab does not take into account any paper, labels, or waste tha ...
... The process of purifying metals by recycling plants has been simplified for the purposes of this lab. For example, during the real process, other materials may be grouped with the aluminum and tin cans, such as plastics. Furthermore, the lab does not take into account any paper, labels, or waste tha ...
Science and the Environment Section 2
... • Ecological footprints are calculations that show the productive area of Earth needed to support one person in a particular country. • An ecological footprint estimates the land used for crops, grazing, forests products, and housing. It also includes the ocean area used to harvest seafood and the f ...
... • Ecological footprints are calculations that show the productive area of Earth needed to support one person in a particular country. • An ecological footprint estimates the land used for crops, grazing, forests products, and housing. It also includes the ocean area used to harvest seafood and the f ...
9/21 properties of matter ppt
... If you were to begin your study of chemistry by looking at substances handy to you, you would most likely encounter more mixtures than anything else. In this section, we will discuss several methods for separating mixtures. As you read about each technique, see how many uses you can identify for it. ...
... If you were to begin your study of chemistry by looking at substances handy to you, you would most likely encounter more mixtures than anything else. In this section, we will discuss several methods for separating mixtures. As you read about each technique, see how many uses you can identify for it. ...
Protein Structure Determined by NMR
... which originate from the interaction of all protons of a spin system that are not directly connected via three chemical bonds. Thus a characteristic pattern of signals results for each amino acid from which the amino acid can be identified ...
... which originate from the interaction of all protons of a spin system that are not directly connected via three chemical bonds. Thus a characteristic pattern of signals results for each amino acid from which the amino acid can be identified ...
CHAPTER 1 CHEMICAL FOUNDATIONS 1 CHAPTER ONE
... a. Distillation separates components of a mixture, so the orange liquid is a mixture (has an average color of the yellow liquid and the red solid). Distillation utilizes boiling point differences to separate out the components of a mixture. Distillation is a physical change because the components of ...
... a. Distillation separates components of a mixture, so the orange liquid is a mixture (has an average color of the yellow liquid and the red solid). Distillation utilizes boiling point differences to separate out the components of a mixture. Distillation is a physical change because the components of ...
PART 2 – CHEMISTRY
... _________ measures, _________ apply to sheep, cattle, goats, pigs and horses, followed confirmation that foot-and-mouth antibodies had been found among British sheep on nine farms and livestock depots in central and northern France. _________ animals were slaughtered last week under stringent measur ...
... _________ measures, _________ apply to sheep, cattle, goats, pigs and horses, followed confirmation that foot-and-mouth antibodies had been found among British sheep on nine farms and livestock depots in central and northern France. _________ animals were slaughtered last week under stringent measur ...
Role of mathematics in chemistry
... branes. These developments, along with the emergence of cheminformatics have resulted in a discipline which is turning out to be very important as fundamental inputs to structural biology and bioinformatics. Control of spatiotemporal patterns via nonlinear systems equations, theoretical electrochemi ...
... branes. These developments, along with the emergence of cheminformatics have resulted in a discipline which is turning out to be very important as fundamental inputs to structural biology and bioinformatics. Control of spatiotemporal patterns via nonlinear systems equations, theoretical electrochemi ...
Pulse Point Newsletter for November, 2005 Published by Alliance
... well as all facility employees on the potential risks of coming in contact with ...
... well as all facility employees on the potential risks of coming in contact with ...
File - Mr. J`s Chemistry 4U
... A- A type of chemical reaction in which two or more substances combine to form a new compound.. B- A type of chemical reaction in which a single compound undergoes a reaction that produces two or more simpler substances. C- A type of chemical reaction in which one element replaces a similar element ...
... A- A type of chemical reaction in which two or more substances combine to form a new compound.. B- A type of chemical reaction in which a single compound undergoes a reaction that produces two or more simpler substances. C- A type of chemical reaction in which one element replaces a similar element ...
atomic number - geraldinescience
... • Physical properties are characteristics that can be observed without changing the composition of the substance. • Physical properties include density, color, hardness, freezing point, boiling point, and the ability to conduct an electric current. • Chemical properties are characteristics that desc ...
... • Physical properties are characteristics that can be observed without changing the composition of the substance. • Physical properties include density, color, hardness, freezing point, boiling point, and the ability to conduct an electric current. • Chemical properties are characteristics that desc ...
Student Activity PDF - TI Education
... 3. For each word equation given on page 2.10, use the Chemical Balance tool on page 2.11 to balance the equation and record it in the table. First, write the balanced equation using the element symbols. Record the number of atoms of each element in the reactant (left side) and the products (right si ...
... 3. For each word equation given on page 2.10, use the Chemical Balance tool on page 2.11 to balance the equation and record it in the table. First, write the balanced equation using the element symbols. Record the number of atoms of each element in the reactant (left side) and the products (right si ...
Volatile Organic Compounds
... memory, reaction time, and hand-eye and foot-eye coordination, and balance and gait disturbances. Exposure can also lead to mood disorders, with depression, irritability, and fatigue being common symptoms. Peripheral neurotoxicity usually results in paresthesias, tremors, and diminished fine and gro ...
... memory, reaction time, and hand-eye and foot-eye coordination, and balance and gait disturbances. Exposure can also lead to mood disorders, with depression, irritability, and fatigue being common symptoms. Peripheral neurotoxicity usually results in paresthesias, tremors, and diminished fine and gro ...
Chapter 9 Stoichiometry
... If everything in the reaction went according to plan, and all of the reactant(s) reacted, this is how much product should be made. This is NOT the same as the actual yield- amount that is produced based on an experiment Error occurs, so actual yield is less than the theoretical yield. Can ne ...
... If everything in the reaction went according to plan, and all of the reactant(s) reacted, this is how much product should be made. This is NOT the same as the actual yield- amount that is produced based on an experiment Error occurs, so actual yield is less than the theoretical yield. Can ne ...
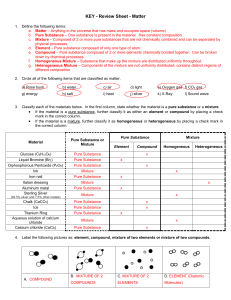
Matter Test Review Sheet
... e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly d ...
... e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly d ...
Chemical Mimicry
... communicate within their own species. It is not surprising that other organisms have evolved the ability to exploit these communication systems in order to fulfill their own needs. ...
... communicate within their own species. It is not surprising that other organisms have evolved the ability to exploit these communication systems in order to fulfill their own needs. ...
Infant formula
... 3.2.1 Milk-based infant formula: refers to liquid or powder products made only through physical methods, of which the main material is milk and milk protein products, supplemented with a proper amount of vitamin, minerals and other supplementary materials, which are applicable to normal infants, whe ...
... 3.2.1 Milk-based infant formula: refers to liquid or powder products made only through physical methods, of which the main material is milk and milk protein products, supplemented with a proper amount of vitamin, minerals and other supplementary materials, which are applicable to normal infants, whe ...
Safety data sheet
A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is an important component of product stewardship and occupational safety and health. It is intended to provide workers and emergency personnel with procedures for handling or working with that substance in a safe manner, and includes information such as physical data (melting point, boiling point, flash point, etc.), toxicity, health effects, first aid, reactivity, storage, disposal, protective equipment, and spill-handling procedures. SDS formats can vary from source to source within a country depending on national requirements.SDSs are a widely used system for cataloging information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product. These data sheets can be found anywhere where chemicals are being used.There is also a duty to properly label substances on the basis of physico-chemical, health and/or environmental risk. Labels can include hazard symbols such as the European Union standard black diagonal cross on an orange background, used to denote a harmful substance.A SDS for a substance is not primarily intended for use by the general consumer, focusing instead on the hazards of working with the material in an occupational setting.In some jurisdictions, the SDS is required to state the chemical's risks, safety, and effect on the environment.It is important to use an SDS specific to both country and supplier, as the same product (e.g. paints sold under identical brand names by the same company) can have different formulations in different countries. The formulation and hazard of a product using a generic name (e.g. sugar soap) may vary between manufacturers in the same country.