Pressure systems
... codes of practice. Since 2002, most pressure equipment placed on the market has had to meet the requirements of the Pressure Equipment Regulations 1999. For pressure equipment not covered by the Pressure Equipment Regulations 1999, the more general requirements of the Pressure Systems Safety Regulat ...
... codes of practice. Since 2002, most pressure equipment placed on the market has had to meet the requirements of the Pressure Equipment Regulations 1999. For pressure equipment not covered by the Pressure Equipment Regulations 1999, the more general requirements of the Pressure Systems Safety Regulat ...
Oobleck Worksheet - Science Education at Jefferson Lab
... • All of your senses, except for taste 2. Open the plastic bag. 3. Carefully measure two teaspoons of the clear liquid into the plastic bag. 4. Next, add two drops of the green liquid. 5. Carefully add two tablespoons of the white liquid. 6. Close the bag and knead the mixture well for 2 minutes. 7. ...
... • All of your senses, except for taste 2. Open the plastic bag. 3. Carefully measure two teaspoons of the clear liquid into the plastic bag. 4. Next, add two drops of the green liquid. 5. Carefully add two tablespoons of the white liquid. 6. Close the bag and knead the mixture well for 2 minutes. 7. ...
CCR Template - Colorado Secretary of State
... SITING OF HAZARDOUS WASTE DISPOSAL SITES 6 CCR 1007-2 Part 2 [Editor’s Notes follow the text of the rules at the end of this CCR Document.] ...
... SITING OF HAZARDOUS WASTE DISPOSAL SITES 6 CCR 1007-2 Part 2 [Editor’s Notes follow the text of the rules at the end of this CCR Document.] ...
Chemical resistance of common metals used in valves. What the
... These tables are not to be used as an absolute recommendation as there are too many factors that can influence the corrosion resistance, such as temperature, temperature fluctuations, concentrations and solutions, velocity and abrasion. J+J therefore accept no responsibility for any problems arising ...
... These tables are not to be used as an absolute recommendation as there are too many factors that can influence the corrosion resistance, such as temperature, temperature fluctuations, concentrations and solutions, velocity and abrasion. J+J therefore accept no responsibility for any problems arising ...
CHEMISTRY SEC 06 SYLLABUS
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
Matter - HCC Learning Web
... Potential & Kinetic Energy • Potential energy, PE, is stored energy; it results from position or composition. PE = mgh • Kinetic energy, KE, is the energy matter has as a result of motion. KE = ½ mv2 • Energy can be converted between the two types. • A boulder at the top of the hill has potential e ...
... Potential & Kinetic Energy • Potential energy, PE, is stored energy; it results from position or composition. PE = mgh • Kinetic energy, KE, is the energy matter has as a result of motion. KE = ½ mv2 • Energy can be converted between the two types. • A boulder at the top of the hill has potential e ...
AS specification - word format File
... e use chemical equations to calculate reacting masses and vice versa using the concepts of amount of substance and molar mass f use chemical equations to calculate volumes of gases and vice versa using the concepts of amount of substance and molar volume of gases, eg calculation of the mass or volum ...
... e use chemical equations to calculate reacting masses and vice versa using the concepts of amount of substance and molar mass f use chemical equations to calculate volumes of gases and vice versa using the concepts of amount of substance and molar volume of gases, eg calculation of the mass or volum ...
CHEMISTRY SEC 06 SYLLABUS
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
CHEMISTRY SEC 06 SYLLABUS
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
CHEMISTRY SEC 06 SYLLABUS
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
... percentage composition by volume of nitrogen and oxygen in air. Air pollution - see Section 5.3(e). Handling techniques for preparation and collection of gases, including the use of the gas syringe. Principle of the extraction of oxygen by fractional distillation of liquid air Preparation of oxygen ...
AP Chemistry Syllabus - Tuloso
... Students and teachers use a recently published (within the last 10 years) collegelevel chemistry textbook. The course is structured around the enduring understandings within the big ideas as described in the AP Chemistry Curriculum Framework. The course provides students with opportunities outside t ...
... Students and teachers use a recently published (within the last 10 years) collegelevel chemistry textbook. The course is structured around the enduring understandings within the big ideas as described in the AP Chemistry Curriculum Framework. The course provides students with opportunities outside t ...
T97008 - Nitric Acid 70% Commercial
... part of a system must be indoors, then care should be taken to isolate from occupied work areas and provide an adequate level of ventilation Low carbon or stabilised stainless steel such as 304L, 315L, 321 or 347 can be used for storage tanks, pipework and fittings at ambient temperature. However on ...
... part of a system must be indoors, then care should be taken to isolate from occupied work areas and provide an adequate level of ventilation Low carbon or stabilised stainless steel such as 304L, 315L, 321 or 347 can be used for storage tanks, pipework and fittings at ambient temperature. However on ...
Grade 10 NSC Chemistry Curriculum
... Ionic structure as illustrated by sodium chloride • Revise the writing of names when given the formulae. • Revise the writing of formulae when given the names • Revise the meaning of the name endings like -ide, -ite and ate • Understand the meaning of prefixes di-, tri- etc • Metallic bonding: - Sha ...
... Ionic structure as illustrated by sodium chloride • Revise the writing of names when given the formulae. • Revise the writing of formulae when given the names • Revise the meaning of the name endings like -ide, -ite and ate • Understand the meaning of prefixes di-, tri- etc • Metallic bonding: - Sha ...
Unit 8 Test Review
... Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another, until all elements are balance ...
... Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another, until all elements are balance ...
o C
... by changing coefficients, never by changing the subscripts in correctly written chemical formulas. ...
... by changing coefficients, never by changing the subscripts in correctly written chemical formulas. ...
Chapter 1: Quiz Review - Wetaskiwin Composite High School
... 6. A chemical bottle carries this WHMIS symbol. What would you expect to happen if you opened the container? A. Escape of the compressed gas B. A burse of flames C. A very bad odour from the escaping chemical fumes D. A sudden rise in temperature as the chemical vaporizes. 7. What is the key identif ...
... 6. A chemical bottle carries this WHMIS symbol. What would you expect to happen if you opened the container? A. Escape of the compressed gas B. A burse of flames C. A very bad odour from the escaping chemical fumes D. A sudden rise in temperature as the chemical vaporizes. 7. What is the key identif ...
Utah - Wavefunction, Inc.
... → Lab 73 "The Process of Dissolving" → Lab 74 "Specifying the Molarity" → Lab 75 "Comparing Molarity and Molality" → Lab 76 "Energetics and Structure of a Dissolved Salt" Objective 3: Differentiate between acids and bases in terms of hydrogen ion concentration. a. Relate hydrogen ion ...
... → Lab 73 "The Process of Dissolving" → Lab 74 "Specifying the Molarity" → Lab 75 "Comparing Molarity and Molality" → Lab 76 "Energetics and Structure of a Dissolved Salt" Objective 3: Differentiate between acids and bases in terms of hydrogen ion concentration. a. Relate hydrogen ion ...
Lecture 14
... Determine the molecular formula of compound that has a molar mass of 78.11 g/mole and an empirical formula of CH. ...
... Determine the molecular formula of compound that has a molar mass of 78.11 g/mole and an empirical formula of CH. ...
Chapter 1 Matter and Energy Classifying Matter – An Exercise
... – units are g/mL (solids and liquids) or g/L (gases) – See Table 1.4 for a listing of densities for common substances ...
... – units are g/mL (solids and liquids) or g/L (gases) – See Table 1.4 for a listing of densities for common substances ...
Contents
... ensure that exactly the correct proportions of the reactants are mixed together so that there is no wastage with some of one reactant being left over. In many processes, in addition to the required product, some waste chemicals are produced. These not only contribute to pollution problems but they a ...
... ensure that exactly the correct proportions of the reactants are mixed together so that there is no wastage with some of one reactant being left over. In many processes, in addition to the required product, some waste chemicals are produced. These not only contribute to pollution problems but they a ...
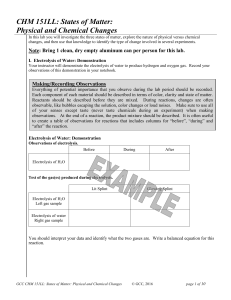
CHM 151LL: States of Matter: Physical and Chemical Changes
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
CSAR Guidance Document
... ‘Specified Person’ may act on their behalf. The HSE must be clear as to the identity of the Specified Person and their relationship to the applicant. In certain circumstances, the HSE may decline to deal with a person seeking to act as a Specified Person. This person may act on behalf of the applica ...
... ‘Specified Person’ may act on their behalf. The HSE must be clear as to the identity of the Specified Person and their relationship to the applicant. In certain circumstances, the HSE may decline to deal with a person seeking to act as a Specified Person. This person may act on behalf of the applica ...
Essential Standard: 8.P.1 Understand the properties of matter and
... Elements are pure substances that cannot be changed into simpler substances. Elements are composed of one kind of atom. Compounds are pure substances that are composed of two or more types of elements that are chemically combined. Compounds can only be changed into simpler substances called elements ...
... Elements are pure substances that cannot be changed into simpler substances. Elements are composed of one kind of atom. Compounds are pure substances that are composed of two or more types of elements that are chemically combined. Compounds can only be changed into simpler substances called elements ...
Diapositive 1 - Aptar
... • Processing aids used during assembly process on machinery or component • Mould release agents • Lubricants • Adhesive, glue, ink from labels or secondary packaging ...
... • Processing aids used during assembly process on machinery or component • Mould release agents • Lubricants • Adhesive, glue, ink from labels or secondary packaging ...
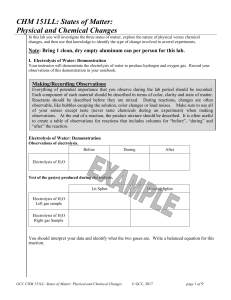
CHM 151LL: States of Matter: Physical and Chemical Changes
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
Safety data sheet
A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is an important component of product stewardship and occupational safety and health. It is intended to provide workers and emergency personnel with procedures for handling or working with that substance in a safe manner, and includes information such as physical data (melting point, boiling point, flash point, etc.), toxicity, health effects, first aid, reactivity, storage, disposal, protective equipment, and spill-handling procedures. SDS formats can vary from source to source within a country depending on national requirements.SDSs are a widely used system for cataloging information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product. These data sheets can be found anywhere where chemicals are being used.There is also a duty to properly label substances on the basis of physico-chemical, health and/or environmental risk. Labels can include hazard symbols such as the European Union standard black diagonal cross on an orange background, used to denote a harmful substance.A SDS for a substance is not primarily intended for use by the general consumer, focusing instead on the hazards of working with the material in an occupational setting.In some jurisdictions, the SDS is required to state the chemical's risks, safety, and effect on the environment.It is important to use an SDS specific to both country and supplier, as the same product (e.g. paints sold under identical brand names by the same company) can have different formulations in different countries. The formulation and hazard of a product using a generic name (e.g. sugar soap) may vary between manufacturers in the same country.