Replicate OPM - MultiscaleLab
... upper right corner, spacing is the distance between dummy atoms. All dummy atoms have the same z. The function returns a Molecule object. Inside the function use mem = Molecule() mem.empty(Ni*Nj) to create an empty molecule with Ni*Nj atoms and then set manually the attributes record, beta, resid, r ...
... upper right corner, spacing is the distance between dummy atoms. All dummy atoms have the same z. The function returns a Molecule object. Inside the function use mem = Molecule() mem.empty(Ni*Nj) to create an empty molecule with Ni*Nj atoms and then set manually the attributes record, beta, resid, r ...
M.Sc. 2015
... Emission without a change in spin multiplicity:is called phosphorescence involves an intersystem crossing is spin forbidden ...
... Emission without a change in spin multiplicity:is called phosphorescence involves an intersystem crossing is spin forbidden ...
Power Point 2 - G. Holmes Braddock
... converted to glucose and metabolized to provide ATP, while others can be stored as fat. Protein is an important compound in controlling fluid volume and osmolality in the blood and body tissues. This function is a major controlling factor in maintaining water balance. Proteins form enzymes that are ...
... converted to glucose and metabolized to provide ATP, while others can be stored as fat. Protein is an important compound in controlling fluid volume and osmolality in the blood and body tissues. This function is a major controlling factor in maintaining water balance. Proteins form enzymes that are ...
-The oxygen consumed during cellular respiration is involved
... -During aerobic respiration, electrons travel downhill in which sequence? -Where are the proteins of the electron transport chain located? -The primary role of oxygen in cellular respiration is to _____. -During aerobic respiration, H2O is formed. Where does the oxygen atom for the formation of the ...
... -During aerobic respiration, electrons travel downhill in which sequence? -Where are the proteins of the electron transport chain located? -The primary role of oxygen in cellular respiration is to _____. -During aerobic respiration, H2O is formed. Where does the oxygen atom for the formation of the ...
Water Drops Template
... by covalent bonds • Hydrogen bonding occurs between polar molecules • An ion is a charged atom or molecule. Ions of opposite charge may form an ionic bond ...
... by covalent bonds • Hydrogen bonding occurs between polar molecules • An ion is a charged atom or molecule. Ions of opposite charge may form an ionic bond ...
Balancing Chemical Reactions
... 1.) In reactions dealing solely with ions, one can leave the polyatomic ions as groups for ease of balancing. 2.) In reactions dealing with only ions and water, water can be considered as a combination of a hydrogen ion and hydroxide ion. 3.) If given a reaction with polyatomic ions that are broken ...
... 1.) In reactions dealing solely with ions, one can leave the polyatomic ions as groups for ease of balancing. 2.) In reactions dealing with only ions and water, water can be considered as a combination of a hydrogen ion and hydroxide ion. 3.) If given a reaction with polyatomic ions that are broken ...
Chapter 3: The Chemistry of Organic Molecules
... • Soluble in water; contains a glycerol molecule, 2 fatty acids, and one phosphate group. • Phosphate group is the “polar head” of molecule. • Fatty acid chains are “nonpolar tails” of molecule. • Plasma membrane in eukaryotic cells is a phospholipid bilayer. ...
... • Soluble in water; contains a glycerol molecule, 2 fatty acids, and one phosphate group. • Phosphate group is the “polar head” of molecule. • Fatty acid chains are “nonpolar tails” of molecule. • Plasma membrane in eukaryotic cells is a phospholipid bilayer. ...
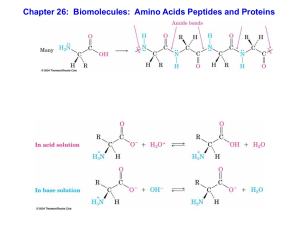
Chapter 26: Biomolecules: Amino Acids Peptides and Proteins
... Contains an imidazole ring that is partially protonated in neutral solution Only the pyridine-like, doubly bonded nitrogen in histidine is basic. The pyrrole-like singly bonded nitrogen is nonbasic because its lone pair of electrons is part of the 6 electron aromatic imidazole ring (see Section 24 ...
... Contains an imidazole ring that is partially protonated in neutral solution Only the pyridine-like, doubly bonded nitrogen in histidine is basic. The pyrrole-like singly bonded nitrogen is nonbasic because its lone pair of electrons is part of the 6 electron aromatic imidazole ring (see Section 24 ...
An ionic bond is a type of chemical bond that involves a metal and a
... ions are more easily polarized, but the effect is usually only important when positive ions with charges of 3+ (e.g., Al3+) are involved. However, 2+ ions (Be2+) or even 1+ (Li+) show some polarizing power because their sizes are so small. ...
... ions are more easily polarized, but the effect is usually only important when positive ions with charges of 3+ (e.g., Al3+) are involved. However, 2+ ions (Be2+) or even 1+ (Li+) show some polarizing power because their sizes are so small. ...
Cellular Respiration Scrambled Steps
... Two things can happen: If oxygen is present, pyruvic acid enters the mitochondria to enter the Kreb’s Cycle. As H+ ions pass back across the mitochondrial membrane through ATP Synthase, molecules of ATP are made. From the Kreb’s cycle, NADH and FADHS enter the electron transport chain. The products ...
... Two things can happen: If oxygen is present, pyruvic acid enters the mitochondria to enter the Kreb’s Cycle. As H+ ions pass back across the mitochondrial membrane through ATP Synthase, molecules of ATP are made. From the Kreb’s cycle, NADH and FADHS enter the electron transport chain. The products ...
Carbon-Based Molecules
... Carbon has unique bonding properties Carbon = building block of life because it makes ...
... Carbon has unique bonding properties Carbon = building block of life because it makes ...
Chapter 1: Electrons and Chemical Bonding
... 3. Columns = oxidation number or valence number, Rows = electron energy level or shell 4. Chemical bonding is the exchange or sharing or electrons to make a fill the outermost energy level with electrons. 5. Lose electron = 1+, gains electron = 16. Ionic bonding is transfer of electrons; covalent bo ...
... 3. Columns = oxidation number or valence number, Rows = electron energy level or shell 4. Chemical bonding is the exchange or sharing or electrons to make a fill the outermost energy level with electrons. 5. Lose electron = 1+, gains electron = 16. Ionic bonding is transfer of electrons; covalent bo ...
ExamView - test.practice.questions.tst
... ____ 25. 4.4 - WWBAT convert between moles & grams What is the mass of 4.7 moles of Na3PO4 (molar mass= 164 grams/mole)? a. 164 g c. 781 g b. 34.9 g d. 542 g ____ 26. 4.4 - WWBAT convert between moles & grams How many moles of carbon-12 are contained in exactly 6 grams of carbon-12? a. 0.5 mole c. m ...
... ____ 25. 4.4 - WWBAT convert between moles & grams What is the mass of 4.7 moles of Na3PO4 (molar mass= 164 grams/mole)? a. 164 g c. 781 g b. 34.9 g d. 542 g ____ 26. 4.4 - WWBAT convert between moles & grams How many moles of carbon-12 are contained in exactly 6 grams of carbon-12? a. 0.5 mole c. m ...
Selection Rules for electronic transitions
... Goals: Use Beers Law to calculate Abs; convert wavelength into wavenumbers; Derive ground state term symbols Upcoming: 11/18, 11/21: Ch. 11. Electronic transitions in metal complexes 11/23 no class 11/28, 11/30: Acc. Chem. Res. 2003, 36, 876-887 Photochemistry for solar energy 12/2: Exam III ...
... Goals: Use Beers Law to calculate Abs; convert wavelength into wavenumbers; Derive ground state term symbols Upcoming: 11/18, 11/21: Ch. 11. Electronic transitions in metal complexes 11/23 no class 11/28, 11/30: Acc. Chem. Res. 2003, 36, 876-887 Photochemistry for solar energy 12/2: Exam III ...
Table of Contents
... A multisubstrate, enzyme-catalyzed reaction in which a product is released before all substrates have been bound to the enzyme is an example of a sequential kinetic mechanism. ...
... A multisubstrate, enzyme-catalyzed reaction in which a product is released before all substrates have been bound to the enzyme is an example of a sequential kinetic mechanism. ...
Exam 1
... B. It consists of an amino group and a carbonyl group C. It consists of an amino group and a carboxyl group D. It consists of a carboxyl group only ____ 17. Which element is most particularly associated with organic chemistry? A. sulfur B. carbon C. nitrogen D. potassium ...
... B. It consists of an amino group and a carbonyl group C. It consists of an amino group and a carboxyl group D. It consists of a carboxyl group only ____ 17. Which element is most particularly associated with organic chemistry? A. sulfur B. carbon C. nitrogen D. potassium ...
A and P Practice Exam 03 (pdf 297.25kb)
... c. if energy is gained by one region of the universe, another place in the universe also must gain energy in order to maintain the balance of nature d. matter tends to become increasingly more disorganized 27. Essentially, the first law of thermodynamics states that __________. a. one form of energy ...
... c. if energy is gained by one region of the universe, another place in the universe also must gain energy in order to maintain the balance of nature d. matter tends to become increasingly more disorganized 27. Essentially, the first law of thermodynamics states that __________. a. one form of energy ...
haemoglobin: structure, properties and biomedical functions
... hemoglobin A. The combination of two alpha chains and two gamma chains form fetal hemoglobin, termed hemoglobin F. The product of the delta globin gene is called hemoglobin A2 The different kinds of chains are encoded for by different genes. The genes that encode the alpha globin chains are on chrom ...
... hemoglobin A. The combination of two alpha chains and two gamma chains form fetal hemoglobin, termed hemoglobin F. The product of the delta globin gene is called hemoglobin A2 The different kinds of chains are encoded for by different genes. The genes that encode the alpha globin chains are on chrom ...
standard sample test
... (c) The solution was found to be neither acidic nor basic, it was neutral. (d) The problem does not have enough information to determine if the solution was found to be acidic, basic or neutral. ...
... (c) The solution was found to be neither acidic nor basic, it was neutral. (d) The problem does not have enough information to determine if the solution was found to be acidic, basic or neutral. ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.