Section 16.3 - CPO Science
... 16.3 Molecules and Carbon Compounds In addition to the elements from which it is made, the shape of a molecule is also important to its function and properties. We use structural diagrams to show the shape and arrangement of atoms in a molecule. ...
... 16.3 Molecules and Carbon Compounds In addition to the elements from which it is made, the shape of a molecule is also important to its function and properties. We use structural diagrams to show the shape and arrangement of atoms in a molecule. ...
Protein Structure Activity
... even a protein called ubiquitin, and it’s in all cells and controls who “lives” and who “dies” among all the proteins in the cell.) All proteins have two things in common: They are all made of chains of building blocks called amino acids. The shape the chain of amino acids folds into is what mak ...
... even a protein called ubiquitin, and it’s in all cells and controls who “lives” and who “dies” among all the proteins in the cell.) All proteins have two things in common: They are all made of chains of building blocks called amino acids. The shape the chain of amino acids folds into is what mak ...
Examination 3 Multiple Choice Questions
... One of the postulates of Dalton’s Atomic Theory states: The atoms of a given element are all alike. In what sense is this true? And, how is this false? The atoms of a given element are all alike in the sense that they have the same number of protons. Atoms of a given element can have different numbe ...
... One of the postulates of Dalton’s Atomic Theory states: The atoms of a given element are all alike. In what sense is this true? And, how is this false? The atoms of a given element are all alike in the sense that they have the same number of protons. Atoms of a given element can have different numbe ...
FE Review Chemistry - UTSA College of Engineering
... a) An element may be separated into atoms b) An element may be a gas, a liquid, or a solid c) A compound can be separated into its elements by chemical means d) An element is always heterogeneous e) A compound may be a gas, a liquid, or a solid 3. In relation to the proton, the electron is a) about ...
... a) An element may be separated into atoms b) An element may be a gas, a liquid, or a solid c) A compound can be separated into its elements by chemical means d) An element is always heterogeneous e) A compound may be a gas, a liquid, or a solid 3. In relation to the proton, the electron is a) about ...
File
... involved in the transport and storage of oxygen • Electron transport and energy metabolism: • Cytochromes are heme-containing compounds serve as electron carriers during the synthesis of ATP • Cytochrome P450 is a family of enzymes important role in the metabolism Shariq AIKC/SYB/2014 ...
... involved in the transport and storage of oxygen • Electron transport and energy metabolism: • Cytochromes are heme-containing compounds serve as electron carriers during the synthesis of ATP • Cytochrome P450 is a family of enzymes important role in the metabolism Shariq AIKC/SYB/2014 ...
3. What are macromolecules?
... worksheet. Color the fatty acid chains the same colors for carbon, hydrogen, and oxygen as you did before. A special type of lipid called phospholipids help make up the cell membrane. Two layers of these phospholipids make up the membrane. Phospholipids have a "water-loving" hydrophilic head and two ...
... worksheet. Color the fatty acid chains the same colors for carbon, hydrogen, and oxygen as you did before. A special type of lipid called phospholipids help make up the cell membrane. Two layers of these phospholipids make up the membrane. Phospholipids have a "water-loving" hydrophilic head and two ...
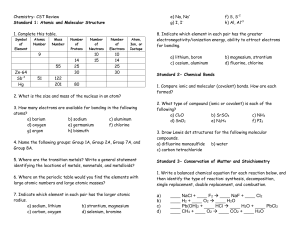
Chemistry- CST Review
... 8. If a sample of gas occupies 6.55 L at 300 °C, what will be its volume at 25 °C if the pressure does not change? 9. A gas at 790 mm Hg and 25 °C occupies a container with an initial volume of 1.20 L. By changing the volume, the pressure of the gas increases to 1500 mm Hg as the temperature is rais ...
... 8. If a sample of gas occupies 6.55 L at 300 °C, what will be its volume at 25 °C if the pressure does not change? 9. A gas at 790 mm Hg and 25 °C occupies a container with an initial volume of 1.20 L. By changing the volume, the pressure of the gas increases to 1500 mm Hg as the temperature is rais ...
Chapter 4: Chemical bonding
... v Explain why potassium oxide does not conduct electricity in the solid state but does in the liquid state. ...
... v Explain why potassium oxide does not conduct electricity in the solid state but does in the liquid state. ...
Chemical Reactions
... The reactants are separated from each other by a plus sign and the products are separated from each other by a plus sign. There should be an arrow in the middle. Examples: When sodium is mixed with water, a purple alkaline solution of sodium hydroxide is produced and hydrogen gas is evolved. Sodium ...
... The reactants are separated from each other by a plus sign and the products are separated from each other by a plus sign. There should be an arrow in the middle. Examples: When sodium is mixed with water, a purple alkaline solution of sodium hydroxide is produced and hydrogen gas is evolved. Sodium ...
Worksheet 8 Notes - Department of Chemistry | Oregon State
... Does Co3+ act as a Lewis acid? Draw the Lewis structure and explain. List and draw two additional Lewis acids. Yes, Co3+ like the transition metal ions acts as Lewis acids. They accept a pair (or several pairs) of electrons to form a new bond (several new bonds). On the left side of the structure be ...
... Does Co3+ act as a Lewis acid? Draw the Lewis structure and explain. List and draw two additional Lewis acids. Yes, Co3+ like the transition metal ions acts as Lewis acids. They accept a pair (or several pairs) of electrons to form a new bond (several new bonds). On the left side of the structure be ...
Summary notes - Kelso High School
... adding an indicator. An indicator is a chemical which changes colour in different pH environments so by matching the colour of the solution to the colour chart, the pH of the substance can be found. The two most commonly used indicators are universal indicator and pH paper, which is like blotting pa ...
... adding an indicator. An indicator is a chemical which changes colour in different pH environments so by matching the colour of the solution to the colour chart, the pH of the substance can be found. The two most commonly used indicators are universal indicator and pH paper, which is like blotting pa ...
RNA and Protein Synthesis
... 1. What does RNA stand for? Ribonucleic Acid 2. What is the sugar in RNA? Ribose 3. What are the three parts of an RNA nucleotide? Nitrogen base, 5-Carbon Sugar, and Phosphate Group 4. What are the three differences between RNA and DNA? The Sugars, (Ribose vs. Deoxyribose,) the nitrogen bases, (U vs ...
... 1. What does RNA stand for? Ribonucleic Acid 2. What is the sugar in RNA? Ribose 3. What are the three parts of an RNA nucleotide? Nitrogen base, 5-Carbon Sugar, and Phosphate Group 4. What are the three differences between RNA and DNA? The Sugars, (Ribose vs. Deoxyribose,) the nitrogen bases, (U vs ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.