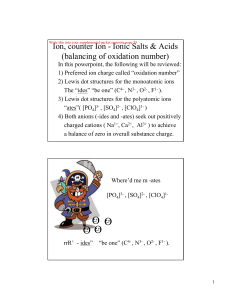
Name________________ Hour____ Chapter 11 Review 1. Name
... 2 atoms of solid aluminum react with 6 molecules of liquid water to produce 3 molecules of hydrogen gas and 2 formula units of solid aluminum hydroxide. 4. Write in symbols: 2 formula units of solid lead (IV) oxide decomposes in the presence of heat to produce 2 formula units of solid lead (II) oxid ...
... 2 atoms of solid aluminum react with 6 molecules of liquid water to produce 3 molecules of hydrogen gas and 2 formula units of solid aluminum hydroxide. 4. Write in symbols: 2 formula units of solid lead (IV) oxide decomposes in the presence of heat to produce 2 formula units of solid lead (II) oxid ...
III. Neutralization
... D12 - Explain the chemical composition of acids and bases, and explain the change of pH in neutralization reactions. ...
... D12 - Explain the chemical composition of acids and bases, and explain the change of pH in neutralization reactions. ...
Homework 5 Insert space or answer ques
... Draw the splitting diagram for a square planar complex Be SURE to identify which d orbitals are located where!! 5.) What is the distinction between a weak field and a strong field ligand? What does it mean for electrons filling the d orbitals? Give an example of a weak field ligand. Give an exampl ...
... Draw the splitting diagram for a square planar complex Be SURE to identify which d orbitals are located where!! 5.) What is the distinction between a weak field and a strong field ligand? What does it mean for electrons filling the d orbitals? Give an example of a weak field ligand. Give an exampl ...
Final Exam Review Answers
... • An atom of an element with atomic number 48 and mass number 120 contains • a. 48 protons, 48 electrons, and 72 neutrons. • b. 72 protons, 48 electrons, and 48 neutrons. • c. 120 protons, 48 electrons, and 72 neutrons. • d. 72 protons, 72 electrons, and 48 neutrons. ...
... • An atom of an element with atomic number 48 and mass number 120 contains • a. 48 protons, 48 electrons, and 72 neutrons. • b. 72 protons, 48 electrons, and 48 neutrons. • c. 120 protons, 48 electrons, and 72 neutrons. • d. 72 protons, 72 electrons, and 48 neutrons. ...
Enzymes - Solon City Schools
... a. SALT: The salt ions interfere with some of the chemical bonds that maintain protein structure b. pH: The same is true of the extra hydrogen ions at very low pH 1. Optimal pH for most enzymes near neutral ...
... a. SALT: The salt ions interfere with some of the chemical bonds that maintain protein structure b. pH: The same is true of the extra hydrogen ions at very low pH 1. Optimal pH for most enzymes near neutral ...
bonds form when water is removed to hold acids together.
... Color code the amino acid on this worksheet (carbon-black, hydrogen-yellow, nitrogen-blue, and oxygen-red). Basic Structure of Amino acid ...
... Color code the amino acid on this worksheet (carbon-black, hydrogen-yellow, nitrogen-blue, and oxygen-red). Basic Structure of Amino acid ...
Chapter 9 Notes: Cellular Respiration
... 1. A calorie is the amount of energy needed to raise the temperature of 1 gram of water 1 degree Celsius 2. Food labels tell you how many calories are in the food products that you eat. The average adult requires about 2000 calories from food each day. B. Cellular Respiration - the process that rele ...
... 1. A calorie is the amount of energy needed to raise the temperature of 1 gram of water 1 degree Celsius 2. Food labels tell you how many calories are in the food products that you eat. The average adult requires about 2000 calories from food each day. B. Cellular Respiration - the process that rele ...
Most common elements in living things are carbon, hydrogen
... functional group (-COOH) is found on the end of the fatty acid that does NOT attach to glycerol. CIRCLE AND LABEL the carboxyl groups in the 2 fatty acids on this worksheet. Color the fatty acid chains the same colors for carbon, hydrogen, and oxygen as you did before. A special type of lipid called ...
... functional group (-COOH) is found on the end of the fatty acid that does NOT attach to glycerol. CIRCLE AND LABEL the carboxyl groups in the 2 fatty acids on this worksheet. Color the fatty acid chains the same colors for carbon, hydrogen, and oxygen as you did before. A special type of lipid called ...
MACROMOLECULE WEBQUEST
... What is the ratio of Carbon to Hydrogen to Oxygen? ________ Carbohydrates comprise what percentage of our body cells? ________ List 4 monosaccharide ...
... What is the ratio of Carbon to Hydrogen to Oxygen? ________ Carbohydrates comprise what percentage of our body cells? ________ List 4 monosaccharide ...
Use of molecular docking to highlight the mechanism of activators
... CA1A2X motif, C is the cysteine residue to which the prenyl group is attached, A1 and A2 are aliphatic amino acids, and X is the carboxyl terminus that specifies which prenyl group is attached. If X is Ala, Cys, Gln, Met, or Ser, the protein is a substrate for FTase and is farnesylated. If X is Leu ...
... CA1A2X motif, C is the cysteine residue to which the prenyl group is attached, A1 and A2 are aliphatic amino acids, and X is the carboxyl terminus that specifies which prenyl group is attached. If X is Ala, Cys, Gln, Met, or Ser, the protein is a substrate for FTase and is farnesylated. If X is Leu ...
Protocol S1.
... parental amino acid sequences and then identifies potentially interacting amino acid pairs based on their proximity (in this case within 4.5 Å) within the resulting folds. The amino acid contact map yielded by this process can then be used to determine the degree of fold disruption expected in any c ...
... parental amino acid sequences and then identifies potentially interacting amino acid pairs based on their proximity (in this case within 4.5 Å) within the resulting folds. The amino acid contact map yielded by this process can then be used to determine the degree of fold disruption expected in any c ...
electrochem1 (2)
... Analyze: We are given a redox equation and asked to identify the substance oxidized and the substance reduced and to label one as the oxidizing agent and the other as the reducing agent. Plan: First, we assign oxidation states to all the atoms in the reaction and determine the elements that are chan ...
... Analyze: We are given a redox equation and asked to identify the substance oxidized and the substance reduced and to label one as the oxidizing agent and the other as the reducing agent. Plan: First, we assign oxidation states to all the atoms in the reaction and determine the elements that are chan ...
Proteins & Nucleic Acids - St. Mary Catholic Secondary School
... with their function – if this shape is not exact in every way, the protein may not function at all. On top of this, if the conditions in which the proteins must function are not just right – the protein may function at a lower capacity or not at all – even if it had the right shape to start. Think o ...
... with their function – if this shape is not exact in every way, the protein may not function at all. On top of this, if the conditions in which the proteins must function are not just right – the protein may function at a lower capacity or not at all – even if it had the right shape to start. Think o ...
Topic 13.2 Periodicity First Row d
... The ions of d-block metals and those in the lower section of the p-block (like lead) have unfilled valence d and p orbitals. These orbitals can accept a lone pair of electrons from species, known as ligands, to form a dative covalent bond between the ligand and the metal ion. Ex. An NH3/H2O (ligands ...
... The ions of d-block metals and those in the lower section of the p-block (like lead) have unfilled valence d and p orbitals. These orbitals can accept a lone pair of electrons from species, known as ligands, to form a dative covalent bond between the ligand and the metal ion. Ex. An NH3/H2O (ligands ...
The Chemistry of Life
... Enzyme- Biological Catalyst Catalyst a chemical agent that changes the rate of a reaction without being consumed by the reaction Enzyme is a catalytic protein. Enzymes provide a way for reactions to occur by lowering the activation energy Activation Energy energy required to get a reaction ...
... Enzyme- Biological Catalyst Catalyst a chemical agent that changes the rate of a reaction without being consumed by the reaction Enzyme is a catalytic protein. Enzymes provide a way for reactions to occur by lowering the activation energy Activation Energy energy required to get a reaction ...
Write this into your supplemental packet opposite page
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
Chemical Biology 03 BLOOD
... ligand; Fe(II) out of plane. • oxyMb, oxyHb ring is flat with sixth ligand bound to Fe(II), metal is in heme plane. ...
... ligand; Fe(II) out of plane. • oxyMb, oxyHb ring is flat with sixth ligand bound to Fe(II), metal is in heme plane. ...
MACROMOLECULE WEBQUEST
... What is the ratio of Carbon to Hydrogen to Oxygen? ________ Carbohydrates comprise what percentage of our body cells? ________ List 4 monosaccharide ...
... What is the ratio of Carbon to Hydrogen to Oxygen? ________ Carbohydrates comprise what percentage of our body cells? ________ List 4 monosaccharide ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.