The Chemical Building Blocks chapt03
... Nucleic Acids (DNA and RNA) • Nucleotides are covalently bound together into long strands through a Dehydration Reaction • The Phosphate of one Nucleotide is bound to the Ribose Sugar of an adjacent nucleotide • These Phosphate-Ribose bonds form the backbones of the Nucleic Acid Molecules ...
... Nucleic Acids (DNA and RNA) • Nucleotides are covalently bound together into long strands through a Dehydration Reaction • The Phosphate of one Nucleotide is bound to the Ribose Sugar of an adjacent nucleotide • These Phosphate-Ribose bonds form the backbones of the Nucleic Acid Molecules ...
24 COORDINATION COMPOUNDS Y MODULE - 6
... Coordination compounds were known in eighteenth century. It was a mystery for the chemist, of those days to understand as to why a stable salt like CoCl3 reacts with varying number of stable molecules or compounds such as ammonia to give several new compounds: CoCl 3.6NH 3, CoCl 3.5NH 3 and CoCl 3.4 ...
... Coordination compounds were known in eighteenth century. It was a mystery for the chemist, of those days to understand as to why a stable salt like CoCl3 reacts with varying number of stable molecules or compounds such as ammonia to give several new compounds: CoCl 3.6NH 3, CoCl 3.5NH 3 and CoCl 3.4 ...
Practice Test 1 (Chapters 1-7)
... added to a beaker of hot water, the water e. none of these immediately turns red. 33. An atom with 15 protons and 16 neutrons is an d. Liquid nitrogen dumped onto the floor atom of vaporizes at room temperature. a. P e. None of the above processes are chemical b. Ga changes. c. S 27. The symbol for ...
... added to a beaker of hot water, the water e. none of these immediately turns red. 33. An atom with 15 protons and 16 neutrons is an d. Liquid nitrogen dumped onto the floor atom of vaporizes at room temperature. a. P e. None of the above processes are chemical b. Ga changes. c. S 27. The symbol for ...
Quiz #3 - San Diego Mesa College
... glucose is _______ by a class of enzymes called ________ . A) reduced ….. dehydrogenases B) oxidized …… dehydrogenases C) oxidized ……. reductases D) reduced …… oxidases E) none of the above Q. 9: Which of the following is/are (a) molecule(s) involved in metabolic redox reactions in living organisms? ...
... glucose is _______ by a class of enzymes called ________ . A) reduced ….. dehydrogenases B) oxidized …… dehydrogenases C) oxidized ……. reductases D) reduced …… oxidases E) none of the above Q. 9: Which of the following is/are (a) molecule(s) involved in metabolic redox reactions in living organisms? ...
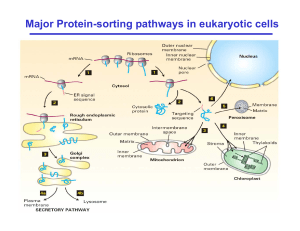
Major Protein-sorting pathways in eukaryotic cells
... proteinsbinding bindingtoto 300-nucleotide 300-nucleotideRNA RNA except p54 except p54 ...
... proteinsbinding bindingtoto 300-nucleotide 300-nucleotideRNA RNA except p54 except p54 ...
CHAPTER 1 -Chemistry -Matter -Elements -Atoms
... 3) Which of the following ions has the same number of electrons as Br(a) Ca+2 (b) K+ (c) Sr+2 (d) I(e) Cl4) For which of the following pairs are the atoms most likely to form an ionic compound? (a) Carbon and Oxygen (b) Calcium and Chlorine (c) Chlorine and Oxygen (d) Sodium and Magnesium (e) Chlori ...
... 3) Which of the following ions has the same number of electrons as Br(a) Ca+2 (b) K+ (c) Sr+2 (d) I(e) Cl4) For which of the following pairs are the atoms most likely to form an ionic compound? (a) Carbon and Oxygen (b) Calcium and Chlorine (c) Chlorine and Oxygen (d) Sodium and Magnesium (e) Chlori ...
Chapter 14 – Inorganic Chemistry
... nCO2 + nH2 O → Cn (H2 O)n + nO2 The reaction is uphill in free energy, and plants use solar energy to carry it out. Photosynthesis is a source of carbohydrates and the only source of oxygen in the atmosphere. However, such reactions have very high activation energies, so nature uses a cluster of fou ...
... nCO2 + nH2 O → Cn (H2 O)n + nO2 The reaction is uphill in free energy, and plants use solar energy to carry it out. Photosynthesis is a source of carbohydrates and the only source of oxygen in the atmosphere. However, such reactions have very high activation energies, so nature uses a cluster of fou ...
Document
... 4. If an amino acid is glucogenic, it will not be degraded to ________. A) pyruvate B) glutamate C) fumarate D) acetoacetate 5. T/F It is possible for an amino acid to be both ketogenic and glucogenic. ...
... 4. If an amino acid is glucogenic, it will not be degraded to ________. A) pyruvate B) glutamate C) fumarate D) acetoacetate 5. T/F It is possible for an amino acid to be both ketogenic and glucogenic. ...
Polypeptide Chain Synthesis: A Paper Simulation
... Involves two amino acids. Involves a dehydration synthesis. Involves a chemical reaction that occurs between two specific areas of the amino acid. Requires an –OH group and an –H from another –OH group ...
... Involves two amino acids. Involves a dehydration synthesis. Involves a chemical reaction that occurs between two specific areas of the amino acid. Requires an –OH group and an –H from another –OH group ...
Amino acid Metabolism 2
... A) serine → glycine B) glutamate → gglycine C) serine → threonine D) glycine → alanine ...
... A) serine → glycine B) glutamate → gglycine C) serine → threonine D) glycine → alanine ...
CHAPTER 3 THE CHEMISTRY OF LIFE Section 1: Matter and
... Each enzyme has an active site, the region where the reaction takes place. The shape of the active site determines which reactants, or substrates, will bind to it. Each different enzyme acts only on specific substrates. Binding of the substrates causes the enzyme’s shape to change. This change cause ...
... Each enzyme has an active site, the region where the reaction takes place. The shape of the active site determines which reactants, or substrates, will bind to it. Each different enzyme acts only on specific substrates. Binding of the substrates causes the enzyme’s shape to change. This change cause ...
Biopolymers
... Three-dimensional structure of B-DNA. The sugar–phosphate backbone winds around the outside of the helix, and the bases occupy the interior. Stacking of the base pairs creates two grooves of unequal width, the major and the minor grooves. In DNA the two strands are wound around each other, joined b ...
... Three-dimensional structure of B-DNA. The sugar–phosphate backbone winds around the outside of the helix, and the bases occupy the interior. Stacking of the base pairs creates two grooves of unequal width, the major and the minor grooves. In DNA the two strands are wound around each other, joined b ...
Name Date AP Biology – Metabolism and Enzymes Review When a
... c. the forward and the backward reactions have stopped. d. ΔG = 0 e. All of the above are true. 6. An endergonic reaction could be described as one that a. proceeds spontaneously with the addition of activation energy. b. produces products with more free energy than the reactants. c. is not able to ...
... c. the forward and the backward reactions have stopped. d. ΔG = 0 e. All of the above are true. 6. An endergonic reaction could be described as one that a. proceeds spontaneously with the addition of activation energy. b. produces products with more free energy than the reactants. c. is not able to ...
CHEMICAL REACTIONS OBJECTIVES 1. To study reactions
... Reactions that evolve heat are called exothermic reactions. In an exothermic reaction, the products have less energy than the reactants. When the products have more energy than the reactants, the reaction is endothermic. The additional energy needed for formation of the products is absorbed from the ...
... Reactions that evolve heat are called exothermic reactions. In an exothermic reaction, the products have less energy than the reactants. When the products have more energy than the reactants, the reaction is endothermic. The additional energy needed for formation of the products is absorbed from the ...
bonding, structure, properties and energy changes
... • An anion is formed when a non-metal atom gains an electron or electrons (monatomic) or when two or more atoms bond covalently with the addition of an electron or electrons (polyatomic). ...
... • An anion is formed when a non-metal atom gains an electron or electrons (monatomic) or when two or more atoms bond covalently with the addition of an electron or electrons (polyatomic). ...
Dinitrogen Cleavage by a Molybdenum(III) Complex
... D.F. Harrison; E. Weissberger; H. Taube. Science, New Series, 1968, 159, 3812, 320-322. Find this online at the ...
... D.F. Harrison; E. Weissberger; H. Taube. Science, New Series, 1968, 159, 3812, 320-322. Find this online at the ...
Chemistry of Transition Metals
... 1. The d-orbitals of the metal interact with the electron cloud of the ligands in such a manner that the d-orbitals become non-degenerate. When the d-level is not completely filled, it is possible to promote and electron from a lower energy d-orbital to a higher energy dorbital by absorption of a ph ...
... 1. The d-orbitals of the metal interact with the electron cloud of the ligands in such a manner that the d-orbitals become non-degenerate. When the d-level is not completely filled, it is possible to promote and electron from a lower energy d-orbital to a higher energy dorbital by absorption of a ph ...
PAP Chemistry - Fall Final Review
... 6. What did Rutherford discover from the Gold Foil Experiment – p.72 The nucleus and that the atom was mostly empty space 7. When is a bright-line spectrum produced by an atom? IE – How does an atom give off color (especially when burned)? The resting state or the ground state is when the electron i ...
... 6. What did Rutherford discover from the Gold Foil Experiment – p.72 The nucleus and that the atom was mostly empty space 7. When is a bright-line spectrum produced by an atom? IE – How does an atom give off color (especially when burned)? The resting state or the ground state is when the electron i ...
Chemical Nomenclature
... This activity is designed to enhance a student’s ability to write and name chemical formulas. The students must be assigned the list of polyatomic ions for memorization. Ideally this memorization is done as a summer assignment or at least a few weeks before this activity. Quiz students over the list ...
... This activity is designed to enhance a student’s ability to write and name chemical formulas. The students must be assigned the list of polyatomic ions for memorization. Ideally this memorization is done as a summer assignment or at least a few weeks before this activity. Quiz students over the list ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.