NCEA Level 2 Chemistry (91165) 2012 Assessment Schedule
... links between the structure, functional groups and the chemical properties of selected organic compounds. This requires the consistent use of chemistry vocabulary, symbols and conventions. ...
... links between the structure, functional groups and the chemical properties of selected organic compounds. This requires the consistent use of chemistry vocabulary, symbols and conventions. ...
NCEA Level 2 Chemistry (91165) 2012
... links between the structure, functional groups and the chemical properties of selected organic compounds. This requires the consistent use of chemistry vocabulary, symbols and conventions. ...
... links between the structure, functional groups and the chemical properties of selected organic compounds. This requires the consistent use of chemistry vocabulary, symbols and conventions. ...
Section 2 Types of Chemical Reactions Chapter 8
... FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) ...
... FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) ...
print
... • The oxidation of a starting aldehyde to a carboxylic acid is achieved using the same aqueous Cr+6 reagents and the mechanism follows steps 2 & 3. ...
... • The oxidation of a starting aldehyde to a carboxylic acid is achieved using the same aqueous Cr+6 reagents and the mechanism follows steps 2 & 3. ...
KUT 203/2 - Chemistry Practical III (Inorganic Chemistry)
... transition metal or metal ion with its oxidation state i.e. VO2+ (yellow), VO2+ (blue) etc. • Determine the composition of a metal complex of which the metal exists in various oxidation states by using the titration technique. • Synthesize several coordination compounds which possess different ligan ...
... transition metal or metal ion with its oxidation state i.e. VO2+ (yellow), VO2+ (blue) etc. • Determine the composition of a metal complex of which the metal exists in various oxidation states by using the titration technique. • Synthesize several coordination compounds which possess different ligan ...
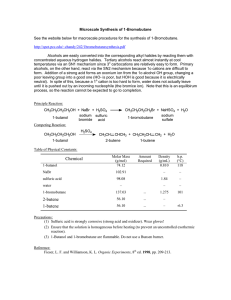
Synthesis of 1
... • Add a boiling stone, pack the neck of the flask with a stainless steel sponge for fractional distillation, and fit it with a dry distilling head and thermometer. Use the Viton connector between the flask and the distilling head. Wrap the column with cotton or glass wool and distill, collecting mat ...
... • Add a boiling stone, pack the neck of the flask with a stainless steel sponge for fractional distillation, and fit it with a dry distilling head and thermometer. Use the Viton connector between the flask and the distilling head. Wrap the column with cotton or glass wool and distill, collecting mat ...
Novel Pharmacotherapy for Treating Cognitive ... Alzheimer’s Disease
... combination difficult to manage, and worsens over time, completely incapacitating the individual. Current therapies modestly manage only one of these medical conditions at a time and a pharmacotherapy that addresses all the above issues without significant side effects is an unmet medical need. In t ...
... combination difficult to manage, and worsens over time, completely incapacitating the individual. Current therapies modestly manage only one of these medical conditions at a time and a pharmacotherapy that addresses all the above issues without significant side effects is an unmet medical need. In t ...
Pincer Complexes. Applications in Catalysis
... As it can be noted from the above, the pincer ligands an their metallic derivatives represent in many cases the ideal examples to carry out catalytic processes otherwise difficult or impossible to be carried out with conventional diphosphine ligands. The versatility of these species to be modified a ...
... As it can be noted from the above, the pincer ligands an their metallic derivatives represent in many cases the ideal examples to carry out catalytic processes otherwise difficult or impossible to be carried out with conventional diphosphine ligands. The versatility of these species to be modified a ...
Worked Example 19.1
... Identify which molecules have been changed and how, starting from the left side of the arrow (the beginning of the reaction) to the right side of the arrow (the end of the reaction). In this case, ethanol is oxidized to acetaldehyde and NAD+ is reduced to NADH/H+. Recognize that nicotinamide adenine ...
... Identify which molecules have been changed and how, starting from the left side of the arrow (the beginning of the reaction) to the right side of the arrow (the end of the reaction). In this case, ethanol is oxidized to acetaldehyde and NAD+ is reduced to NADH/H+. Recognize that nicotinamide adenine ...
Unit 4 - INTEC Chemistry Blog
... Redox (reduction oxidation reaction) describes / all chemical reactions in which atoms have their oxidation number/state changed Oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic Oxidation describes the loss of electrons by ...
... Redox (reduction oxidation reaction) describes / all chemical reactions in which atoms have their oxidation number/state changed Oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic Oxidation describes the loss of electrons by ...
American-Journal-of-Oil-and-Chemical-Technologies
... A solution of H2pydco (0.037 g, 0.2 mmol) in water (10 ml) was added dropwise to a solution of 1,10phenanthroline (0.039 g, 0.2 mmol) in water (5 ml) and the mixture was stirred at room temperature for 2h. Then, a solution of CdCl2·2H2O (0.043 g, 0.2 mmol) in water (5 ml) is added to the reaction mi ...
... A solution of H2pydco (0.037 g, 0.2 mmol) in water (10 ml) was added dropwise to a solution of 1,10phenanthroline (0.039 g, 0.2 mmol) in water (5 ml) and the mixture was stirred at room temperature for 2h. Then, a solution of CdCl2·2H2O (0.043 g, 0.2 mmol) in water (5 ml) is added to the reaction mi ...
Topic 20 Organic Chemistry
... Identify the feature which both molecules possess that accounts for this property. When 2-hydroxypropanoic acid is formed from 2-chloropropanoic acid, the product shows no optical activity. Deduce the type of nucleophilic substitution that takes place and explain your answer. ...
... Identify the feature which both molecules possess that accounts for this property. When 2-hydroxypropanoic acid is formed from 2-chloropropanoic acid, the product shows no optical activity. Deduce the type of nucleophilic substitution that takes place and explain your answer. ...
Lecture Resource ()
... Irene Lee Case Western Reserve University Cleveland, OH ©2004, Prentice Hall ...
... Irene Lee Case Western Reserve University Cleveland, OH ©2004, Prentice Hall ...
CH343 Advanced Organic Chemistry
... I expect you to ask me, a classmate, a tutor, or somebody for help when you do not understand a topic or a problem. I expect you to try your best to learn organic chemistry, and I promise I will try my best to help you learn organic chemistry and to get the most out of this class that you can in ter ...
... I expect you to ask me, a classmate, a tutor, or somebody for help when you do not understand a topic or a problem. I expect you to try your best to learn organic chemistry, and I promise I will try my best to help you learn organic chemistry and to get the most out of this class that you can in ter ...
Chabot College
... Before entering the course, the student should be able to: 1. name compounds of the common organic functional groups using the IUPAC system of nomenclature; 2. use a mechanistic approach to make reasonable predictions of major products formed in reactions involving hydrocarbons, alkyl halides, alcoh ...
... Before entering the course, the student should be able to: 1. name compounds of the common organic functional groups using the IUPAC system of nomenclature; 2. use a mechanistic approach to make reasonable predictions of major products formed in reactions involving hydrocarbons, alkyl halides, alcoh ...
Theoretical Competition - Austrian Chemistry Olympiad
... Substance I crystallises in a NaCl-structure and contains two elements. Compound J is found in nature. It is used in huge amounts as extender in the paper industry. The synthesis of I from J takes place in a rotary furnace at 1000 – 1200°C by reduction with coke, whereby carbon oxide is produced. ...
... Substance I crystallises in a NaCl-structure and contains two elements. Compound J is found in nature. It is used in huge amounts as extender in the paper industry. The synthesis of I from J takes place in a rotary furnace at 1000 – 1200°C by reduction with coke, whereby carbon oxide is produced. ...
- Wiley Online Library
... The ORCID identification number for an author of this article can be found under http://dx.doi.org/10.1002/anie.201606701. ...
... The ORCID identification number for an author of this article can be found under http://dx.doi.org/10.1002/anie.201606701. ...
CHEMICAL REACTIONS
... ________ 21. Double-replacement reactions are generally driven by the formation of: a. a precipitate. c. water. b. a gaseous product. d. all of the above ________ 22. If butane (C4H10) undergoes complete combustion: a. 8 CO2 is one product. c. 9 O2 is one reactant. b. 8 CO is one product. d. 10 O2 i ...
... ________ 21. Double-replacement reactions are generally driven by the formation of: a. a precipitate. c. water. b. a gaseous product. d. all of the above ________ 22. If butane (C4H10) undergoes complete combustion: a. 8 CO2 is one product. c. 9 O2 is one reactant. b. 8 CO is one product. d. 10 O2 i ...
Name__________________________Review Organic Reactions
... 1. Given the incomplete equation representing an organic addition reaction: X(g) + Cl 2(g) XCl2(g) Which compound could be represented by X? A) CH 4 C) C3H8 ...
... 1. Given the incomplete equation representing an organic addition reaction: X(g) + Cl 2(g) XCl2(g) Which compound could be represented by X? A) CH 4 C) C3H8 ...
Faculteit der Natuurwetenschappen, Wiskunde en Informatica
... a [2+2] photocycloaddition. A challenging part of this approach is to obtain enantiomerically pure β-hydroxy-ketones (17, figure 1), which are required for the synthesis of the photo substrate (1). Chiral acetals are used to obtain β-hydroxy-ketones via diastereoselective addition of Grignard reagen ...
... a [2+2] photocycloaddition. A challenging part of this approach is to obtain enantiomerically pure β-hydroxy-ketones (17, figure 1), which are required for the synthesis of the photo substrate (1). Chiral acetals are used to obtain β-hydroxy-ketones via diastereoselective addition of Grignard reagen ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.