(Marine Bioinorganic Chemistry) 12.755 Lecture 2
... Ligands can include inorganic chemical species: In oxic systems: OH-, CO32-,SO42-, Cl-, PO43-, In anoxic systems add: HS-,, S2Ligands can also include organic chemical species: EDTA, DTPA, NTA, Citrate, Tris, siderophores, cobalophores, DFB, TETA, and the famous unknown ligand(s) “L” ...
... Ligands can include inorganic chemical species: In oxic systems: OH-, CO32-,SO42-, Cl-, PO43-, In anoxic systems add: HS-,, S2Ligands can also include organic chemical species: EDTA, DTPA, NTA, Citrate, Tris, siderophores, cobalophores, DFB, TETA, and the famous unknown ligand(s) “L” ...
Lecture 14. Chemistry of Groups I, II, and III
... Hydrogen is the simplest element, consisting of a single proton and electron. It has a reasonably high electronegativity, which means that it forms covalent bonds with carbon which has a similar electronegativity of 2.5. Once it has ionized to form a proton, it has no remaining electrons, and, in th ...
... Hydrogen is the simplest element, consisting of a single proton and electron. It has a reasonably high electronegativity, which means that it forms covalent bonds with carbon which has a similar electronegativity of 2.5. Once it has ionized to form a proton, it has no remaining electrons, and, in th ...
Oxidation of alcohols and aldehydes
... Further oxidation of a primary alcohol • Using a process known as REFLUX, the reaction contents are continually heated at their boiling point temperature, so HOTTER and LONGER heating then alcohol conversion to an aldehyde • Still uses acidified potassium dichromate Primary + Oxidising Carboxylic ...
... Further oxidation of a primary alcohol • Using a process known as REFLUX, the reaction contents are continually heated at their boiling point temperature, so HOTTER and LONGER heating then alcohol conversion to an aldehyde • Still uses acidified potassium dichromate Primary + Oxidising Carboxylic ...
Available - Ggu.ac.in
... of the splitting Δ that they produce (small Δ to large Δ): I− < Br− < S2− < SCN− < Cl− < NO3− < N3− < F− < OH− < C2O42− < H2O < NCS− < CH3CN < py < NH3 < en < 2,2'-bipyridine< phen < NO2− < PPh3 < CN− < CO It is useful to note that the ligands producing the most splitting are those that can engage i ...
... of the splitting Δ that they produce (small Δ to large Δ): I− < Br− < S2− < SCN− < Cl− < NO3− < N3− < F− < OH− < C2O42− < H2O < NCS− < CH3CN < py < NH3 < en < 2,2'-bipyridine< phen < NO2− < PPh3 < CN− < CO It is useful to note that the ligands producing the most splitting are those that can engage i ...
5.04 Principles of Inorganic Chemistry II
... 5.04, Principles of Inorganic Chemistry II Prof. Daniel G. Nocera Lecture 10: General Electronic Considerations of Metal-Ligand Complexes Metal complexes are Lewis acid-base adducts formed between metal ions (the acid) and ligands (the base). ...
... 5.04, Principles of Inorganic Chemistry II Prof. Daniel G. Nocera Lecture 10: General Electronic Considerations of Metal-Ligand Complexes Metal complexes are Lewis acid-base adducts formed between metal ions (the acid) and ligands (the base). ...
Chapter 9: Coordination Compounds
... 23. Structural isomerism: This type of isomerism arises due to the difference in structures of coordination compounds. Structural isomerism, or constitutional isomerism, is a form of isomerism in which molecules with the same molecular formula have atoms bonded together in different orders. a. Ionis ...
... 23. Structural isomerism: This type of isomerism arises due to the difference in structures of coordination compounds. Structural isomerism, or constitutional isomerism, is a form of isomerism in which molecules with the same molecular formula have atoms bonded together in different orders. a. Ionis ...
Lecture 8 - The Spectrochemical Series – Color and Magnetism 1
... splitting energy is different when chloride is a ligand than when water is a ligand. ...
... splitting energy is different when chloride is a ligand than when water is a ligand. ...
Chapter 1 Structure and Bonding
... iii. CoA4B2 only has two isomers. Trigonal Prismatic and Trigonal Antiprimatic should give 3 isomers. Octahedral only has two possible isomers. Thus the structure must be Oh. iv. PtA2B2 only has two isomers. It must be square planar rather than tetrahedral, which would only have 1 isomer. v. ...
... iii. CoA4B2 only has two isomers. Trigonal Prismatic and Trigonal Antiprimatic should give 3 isomers. Octahedral only has two possible isomers. Thus the structure must be Oh. iv. PtA2B2 only has two isomers. It must be square planar rather than tetrahedral, which would only have 1 isomer. v. ...
Organometallic Chemistry
... Agostic – derived from Greek word for "to hold on to oneself” C-H bond on a ligand that undergoes an interaction with the metal complex resembles the transition state of an oxidative addition or reductive elimination reaction. Detected by NMR spectroscopy, X-ray diffraction Compound above: Mo–H = ...
... Agostic – derived from Greek word for "to hold on to oneself” C-H bond on a ligand that undergoes an interaction with the metal complex resembles the transition state of an oxidative addition or reductive elimination reaction. Detected by NMR spectroscopy, X-ray diffraction Compound above: Mo–H = ...
Metal Complex Isomers
... important in the development of structure-property relationships of transition metal complexes, especially in catalysis and in metallobiological compounds. Current FDA policy requires that when optical isomers of pharmacologically active compounds are possible, they must be separated and tested sepa ...
... important in the development of structure-property relationships of transition metal complexes, especially in catalysis and in metallobiological compounds. Current FDA policy requires that when optical isomers of pharmacologically active compounds are possible, they must be separated and tested sepa ...
(Marine Bioinorganic Chemistry) 12.755 Lecture 2
... Solubility Products: Example for Fe(OH)3(s) Ksp= [Fe][OH]3 = 1042.7 Stability constants for metal complexes (where L is ligand, M is Metal): K = [ML]/[M][L] Ligands can include inorganic chemical species: In oxic systems: OH-, CO32-,SO42-, Cl-, PO43-, In anoxic systems add: HS-,, S2Ligands can also ...
... Solubility Products: Example for Fe(OH)3(s) Ksp= [Fe][OH]3 = 1042.7 Stability constants for metal complexes (where L is ligand, M is Metal): K = [ML]/[M][L] Ligands can include inorganic chemical species: In oxic systems: OH-, CO32-,SO42-, Cl-, PO43-, In anoxic systems add: HS-,, S2Ligands can also ...
Part I- unit IV Coord Chem
... Transition Metals (incomplete d-orbitals) Transition-metal compounds are often colored Transition metals form complex ions. Some complexes are neutral. A complex ion is a metal ion with Lewis bases attached to it through coordinate covalent bonds. Ligands are the Lewis bases attached to the metal at ...
... Transition Metals (incomplete d-orbitals) Transition-metal compounds are often colored Transition metals form complex ions. Some complexes are neutral. A complex ion is a metal ion with Lewis bases attached to it through coordinate covalent bonds. Ligands are the Lewis bases attached to the metal at ...
Chapter 1 Structure and Bonding
... Alcohol Synthesis by Reduction of Aldehydes and Ketones 1) Hydrogenation = adding H2 to a double bond H H ...
... Alcohol Synthesis by Reduction of Aldehydes and Ketones 1) Hydrogenation = adding H2 to a double bond H H ...
Transition Metal Complexes
... 2. The ligand names are given in alphabetical order. Some ligands have familiar names also used in naming other types of compounds (eg. chloro, cyano); others have names special to complexes (eg. carbonato, CO32-; aqua, H2O). 3. The name of the central metal atom or ion followed by its oxidation sta ...
... 2. The ligand names are given in alphabetical order. Some ligands have familiar names also used in naming other types of compounds (eg. chloro, cyano); others have names special to complexes (eg. carbonato, CO32-; aqua, H2O). 3. The name of the central metal atom or ion followed by its oxidation sta ...
Coordination Number 2
... Answer: tris(ethylenediamine)cobalt(III) sulfate Solution: The sulfate is the counter anion in this molecule. Since it takes 3 sulfates to bond with two complex cations, the charge on each complex cation must be +3. Since ethylenediamine is a neutral molecule, the oxidation number of cobalt in the c ...
... Answer: tris(ethylenediamine)cobalt(III) sulfate Solution: The sulfate is the counter anion in this molecule. Since it takes 3 sulfates to bond with two complex cations, the charge on each complex cation must be +3. Since ethylenediamine is a neutral molecule, the oxidation number of cobalt in the c ...
Chemistry of Transition Metals
... with metal ions in the center of the cube. The ligands occupy the four alternate corners of the cube leaving the rest four corners empty. The two ‘e’ orbitals point to the center of the face of the cube while the three ‘t2’ orbitals point to the center of the edges of the cube. Therefore, the angle ...
... with metal ions in the center of the cube. The ligands occupy the four alternate corners of the cube leaving the rest four corners empty. The two ‘e’ orbitals point to the center of the face of the cube while the three ‘t2’ orbitals point to the center of the edges of the cube. Therefore, the angle ...
Exercises_Exam_II_material
... (2) In IF5 solvent, SbF5 acts as an acid while KF acts as a base. Explain and write balanced chemical equations to justify your answer. (3) HCN behaves as an acid with diethyl ether but as a base with SbF5. Explain and sketch suitable structures to justify your answer. (4) Among group 12 metals, zin ...
... (2) In IF5 solvent, SbF5 acts as an acid while KF acts as a base. Explain and write balanced chemical equations to justify your answer. (3) HCN behaves as an acid with diethyl ether but as a base with SbF5. Explain and sketch suitable structures to justify your answer. (4) Among group 12 metals, zin ...
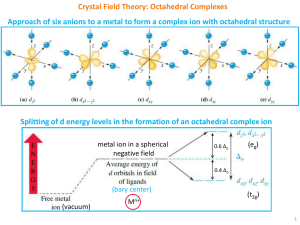
Crystal Field Theory: Octahedral Complexes
... CFSE for (t2g)x(eg)y configuration = (0.4x - 0.6y)Δo (ignoring pairing energy) For d4 configuration, the size of the gap (Δo) will determine whether the fourth electron enters the lower t2g set of orbitals, or the upper eg set of orbitals. The outcome will depend on the relative size of the spli ...
... CFSE for (t2g)x(eg)y configuration = (0.4x - 0.6y)Δo (ignoring pairing energy) For d4 configuration, the size of the gap (Δo) will determine whether the fourth electron enters the lower t2g set of orbitals, or the upper eg set of orbitals. The outcome will depend on the relative size of the spli ...
Chapter 17 Aldehydes and Ketones
... parent alkane to -al. • Because the carbonyl group of an aldehyde can only be at the end of a parent chain and numbering must start with it as carbon-1, there is no need to use a number to locate the aldehyde group. • For unsaturated aldehydes, indicate the presence of a carbon-carbon double bond by ...
... parent alkane to -al. • Because the carbonyl group of an aldehyde can only be at the end of a parent chain and numbering must start with it as carbon-1, there is no need to use a number to locate the aldehyde group. • For unsaturated aldehydes, indicate the presence of a carbon-carbon double bond by ...
Microsoft Word
... and stereo defined stable enolates” (e.g., irreversibly generated metal enolates such as silyl, borane, titanium, and tin enolates) to “in situ formed labile enolate synthons” There have been numerous reports on small-molecule catalyzed direct aldol reactions using the latter enolate equivalents. In ...
... and stereo defined stable enolates” (e.g., irreversibly generated metal enolates such as silyl, borane, titanium, and tin enolates) to “in situ formed labile enolate synthons” There have been numerous reports on small-molecule catalyzed direct aldol reactions using the latter enolate equivalents. In ...
allowed transitions: g $ u forbidden transitions
... to about 8000 cm_1 as X is varied from Cl to Br to I . Metal to Ligand( M L ): A transfer of charge of electrons from metal to ligands is most commonly observed in complexes with ligands that have low lying π* orbitals, especially carbonyl ( CO ) , cyanide ( CN ) and aromatic ligands ( diimine,, phe ...
... to about 8000 cm_1 as X is varied from Cl to Br to I . Metal to Ligand( M L ): A transfer of charge of electrons from metal to ligands is most commonly observed in complexes with ligands that have low lying π* orbitals, especially carbonyl ( CO ) , cyanide ( CN ) and aromatic ligands ( diimine,, phe ...
Chapter 2 - U of L Class Index
... Note that different ligands provide different amounts of crystal field splitting. Fe(OH2)63+ and Fe(C2O4)33- are both complexes of Fe3+ but Fe(OH2)63+ is red-orange while Fe(C2O4)33- is green. A spectrophotometer measures the amount of light absorbed by a complex. When analyzing a green complex, it ...
... Note that different ligands provide different amounts of crystal field splitting. Fe(OH2)63+ and Fe(C2O4)33- are both complexes of Fe3+ but Fe(OH2)63+ is red-orange while Fe(C2O4)33- is green. A spectrophotometer measures the amount of light absorbed by a complex. When analyzing a green complex, it ...
Slide 1
... Prof. Standshort’s organmoetallics class), noticed this and suggested that Fred use the exact same conditions except that he should use PhCH2MgBr (benzyl Grignard) instead of EtMgBr. Fred frantically did so and found that the reaction now gave a quantitative yield of orange Pd(CH2Ph)2(PMe3)2. Why di ...
... Prof. Standshort’s organmoetallics class), noticed this and suggested that Fred use the exact same conditions except that he should use PhCH2MgBr (benzyl Grignard) instead of EtMgBr. Fred frantically did so and found that the reaction now gave a quantitative yield of orange Pd(CH2Ph)2(PMe3)2. Why di ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.