
Coordination Chemistry and Ligand Exchange reactions in the
... initial dissociation of one or two triphenylphosphine ligands to give 14 or 12electron complex followed by oxidative addition of H2 to the metal. Subsequent πcomplexation of alkene, intramolecular hydride transfer (olefin insertion), and reductive elimination forming the alkane. ...
... initial dissociation of one or two triphenylphosphine ligands to give 14 or 12electron complex followed by oxidative addition of H2 to the metal. Subsequent πcomplexation of alkene, intramolecular hydride transfer (olefin insertion), and reductive elimination forming the alkane. ...
Thermochemistry (4 lectures)
... Ligand substitution reactions • Dissociative and associative mechanisms possible • Rates vary widely for transition metal complexes M3+ slower than M2+ dn with large LFSE are slow (d3, d8 and low spin d5-7) Today’s lecture • e- transfer reactions ...
... Ligand substitution reactions • Dissociative and associative mechanisms possible • Rates vary widely for transition metal complexes M3+ slower than M2+ dn with large LFSE are slow (d3, d8 and low spin d5-7) Today’s lecture • e- transfer reactions ...
Organic Chemistry/Fourth Edition: e-Text
... Ethyl isopropyl ketone may be alternatively named 2-methyl-3-pentanone. Its longest continuous chain has five carbons. The carbonyl carbon is C-3 irrespective of the direction in which the chain is numbered, and so we choose the direction that gives the lower number to the position that bears the me ...
... Ethyl isopropyl ketone may be alternatively named 2-methyl-3-pentanone. Its longest continuous chain has five carbons. The carbonyl carbon is C-3 irrespective of the direction in which the chain is numbered, and so we choose the direction that gives the lower number to the position that bears the me ...
Fe(H 2 O) 6 2+ + Fe(H 2 O) 6 3+ *Fe(H 2 O) 6 3+ + Fe(H 2 O) 6 2+
... Ligand substitution reactions • Dissociative and associative mechanisms possible • Rates vary widely for transition metal complexes M3+ slower than M2+ dn with large LFSE are slow (d3, d8 and low spin d5-7) Today’s lecture • e- transfer reactions ...
... Ligand substitution reactions • Dissociative and associative mechanisms possible • Rates vary widely for transition metal complexes M3+ slower than M2+ dn with large LFSE are slow (d3, d8 and low spin d5-7) Today’s lecture • e- transfer reactions ...
Structural and Functional Aspects of Metal Sites in Biology
... expected to vary by about 1 log unit in proteins, owing to dielectric and local electrostatic effects (except if a protein conformational change is coupled to a deprotonation reaction).8 Metals can of course bind ligands at pH values well below their pKa’s. For example, coordination at the unprotona ...
... expected to vary by about 1 log unit in proteins, owing to dielectric and local electrostatic effects (except if a protein conformational change is coupled to a deprotonation reaction).8 Metals can of course bind ligands at pH values well below their pKa’s. For example, coordination at the unprotona ...
investigation of the mechanism of phosphotriesterase
... Electron paramagnetic resonance spectroscopy (EPR) is the major technique applied in this investigation of the binuclear active site of Mn(II)-substituted PTE. The theory and use of EPR in the study of binuclear enzymes will be discussed in Chapter II. Organophosphates contribute to half of all pest ...
... Electron paramagnetic resonance spectroscopy (EPR) is the major technique applied in this investigation of the binuclear active site of Mn(II)-substituted PTE. The theory and use of EPR in the study of binuclear enzymes will be discussed in Chapter II. Organophosphates contribute to half of all pest ...
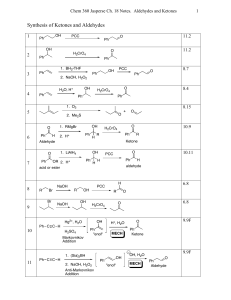
Synthesis of Ketones and Aldehydes
... • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict what product forms that can be isolated, you will need to know when an add ...
... • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict what product forms that can be isolated, you will need to know when an add ...
6149.pdf
... TBP–HNO3 complex at high pressures could lead to enhanced corrosion. Therefore, there is a need to develop a new supercritical fluid extraction process, obviating the usage of HNO3 . Since supercritical carbon dioxide (SC CO2 ) is a non-solvent for ionic species, suitable modifiers or coordinating lig ...
... TBP–HNO3 complex at high pressures could lead to enhanced corrosion. Therefore, there is a need to develop a new supercritical fluid extraction process, obviating the usage of HNO3 . Since supercritical carbon dioxide (SC CO2 ) is a non-solvent for ionic species, suitable modifiers or coordinating lig ...
The mineralogy and chemistry of the nickel carbonates
... carbonate was produced in any of these runs, nickel hydroxy-salts being obtained instead. I t is significant t h a t while anhydrous nickel carbonate has never been found as a natural mineral, it can be made with relative ease from zararite. A sample of natural zaratite was heated to 225 ~ C in a bo ...
... carbonate was produced in any of these runs, nickel hydroxy-salts being obtained instead. I t is significant t h a t while anhydrous nickel carbonate has never been found as a natural mineral, it can be made with relative ease from zararite. A sample of natural zaratite was heated to 225 ~ C in a bo ...
Fragmentation and charge transfer in gas
... or collisional heating in the source prior to mass-spectrometric analysis. This route obviously circumvents the smallest complexes in which spontaneous dissociative charge transfer would occur. Most subsequent work has concentrated on aqueous metal ions [7±13]. However, ESI can generate complexes in ...
... or collisional heating in the source prior to mass-spectrometric analysis. This route obviously circumvents the smallest complexes in which spontaneous dissociative charge transfer would occur. Most subsequent work has concentrated on aqueous metal ions [7±13]. However, ESI can generate complexes in ...
The Impact of Ligand Design on the Coordination Chemistry and
... Second, for carbonylrhodium(I) complexes, (NNN)Rh(CO), substitution at the para-aryl positions predictably modulates the electronic properties and chemical reactivity. Oxidative addition reactions of the (NNN)Rh(CO) with iodoalkanes proceed about three orders of magnitude faster than those reported ...
... Second, for carbonylrhodium(I) complexes, (NNN)Rh(CO), substitution at the para-aryl positions predictably modulates the electronic properties and chemical reactivity. Oxidative addition reactions of the (NNN)Rh(CO) with iodoalkanes proceed about three orders of magnitude faster than those reported ...
A Metal-Based Inhibitor of Tumor Necrosis Factor-α
... towards the TNF-α dimer, we first used molecular modeling to analyze the interaction of Δ-1 and Λ-1 with TNF-α using the X-ray co-crystal structure of the TNF-α dimer with SPD304 (PDB code: 2AZ5).[8] The binding interaction was evaluated using the Molsoft ICM method [ICM-Pro 3.6-1d molecular docking ...
... towards the TNF-α dimer, we first used molecular modeling to analyze the interaction of Δ-1 and Λ-1 with TNF-α using the X-ray co-crystal structure of the TNF-α dimer with SPD304 (PDB code: 2AZ5).[8] The binding interaction was evaluated using the Molsoft ICM method [ICM-Pro 3.6-1d molecular docking ...
Carbon dioxide and metal centres: from reactions
... D. Walther et al. / Coordination Chemistry Re6iews 182 (1999) 67–100 ...
... D. Walther et al. / Coordination Chemistry Re6iews 182 (1999) 67–100 ...
A Chapter 4 Organometallics
... d and f organometallic compounds are particularly sensitive to oxygen and water and this problem is more important for f-elements in view of their larger ionic radii and their lower propensity to form covalent bonds. For f-elements, relativistic effects (see Ch. 1) increase the complexity of theoret ...
... d and f organometallic compounds are particularly sensitive to oxygen and water and this problem is more important for f-elements in view of their larger ionic radii and their lower propensity to form covalent bonds. For f-elements, relativistic effects (see Ch. 1) increase the complexity of theoret ...
Latest Publication (still not complete)
... metals in low oxidation states. The most common Fischer carbene complexes are those of the group 6 transition metals (Cr, Mo, W), in which the metal moiety exists in a zero oxidation state. However, more important is the fact that the carbene moiety in such complexes is electrophilic, hence it tends ...
... metals in low oxidation states. The most common Fischer carbene complexes are those of the group 6 transition metals (Cr, Mo, W), in which the metal moiety exists in a zero oxidation state. However, more important is the fact that the carbene moiety in such complexes is electrophilic, hence it tends ...
Lecture 1: Key Concepts in Stereoselective Synthesis
... -If Lewis acid can catalyze allylboration without interfering the chair-like 6-membered cyclic transition state, the reaction would allow a catalytic, enantioselective, regiospecific and diastereospecific approach to chiral ...
... -If Lewis acid can catalyze allylboration without interfering the chair-like 6-membered cyclic transition state, the reaction would allow a catalytic, enantioselective, regiospecific and diastereospecific approach to chiral ...
Iridium(III) and Rhodium(III) compounds of dipyridyl-N
... in coordination of the transformed ligand, (C5 H4 N)2 C=NH to the metal centre. Complexes were obtained as their hexafluorophosphate salts and characterized based on IR, NMR and ESI-MS spectroscopic data. Authenticity of NH-ketimine organometallic compound was established by single crystal X-ray anal ...
... in coordination of the transformed ligand, (C5 H4 N)2 C=NH to the metal centre. Complexes were obtained as their hexafluorophosphate salts and characterized based on IR, NMR and ESI-MS spectroscopic data. Authenticity of NH-ketimine organometallic compound was established by single crystal X-ray anal ...
The solid state and solution structures of tin(IV)
... that have been prepared, their physical properties, and molecular complexities are given in Table 1. It is noteworthy that the molecular complexities determined in solution may have been measured under different conditions and therefore may not be strictly comparable. There are also some contradicto ...
... that have been prepared, their physical properties, and molecular complexities are given in Table 1. It is noteworthy that the molecular complexities determined in solution may have been measured under different conditions and therefore may not be strictly comparable. There are also some contradicto ...
CHM 103 Lecture 24 S07
... • contains an ─O─ between two carbon groups (R-O-R’). • has a common name that gives the alkyl names of the attached groups followed by ether. CH3─O─CH3 CH3─CH2─O─CH3 ...
... • contains an ─O─ between two carbon groups (R-O-R’). • has a common name that gives the alkyl names of the attached groups followed by ether. CH3─O─CH3 CH3─CH2─O─CH3 ...
Nitrogen Complexes of the Platinum Metals
... Despite reports to the contrary, the nitrogen ligand has not been reduced to ammonia in any complex (4). So far only the Group VIII metals are known to form nitrogen complexes, and those of cobalt, ruthenium and iridium have received most study. This article is not concerned with nitrogen complexes ...
... Despite reports to the contrary, the nitrogen ligand has not been reduced to ammonia in any complex (4). So far only the Group VIII metals are known to form nitrogen complexes, and those of cobalt, ruthenium and iridium have received most study. This article is not concerned with nitrogen complexes ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.


















![Imidazole-Based [2 + 1] Re(I)/99mTc(I](http://s1.studyres.com/store/data/016453198_1-7284afe0756f420c3d5e168652a9a305-300x300.png)
