AP Unit 1 Test Review
... 7. All of the following statements concerning the characteristics of the halogens are true EXCEPT: (A) The first ionization energies (potentials) decrease as the atomic numbers of the halogens increase. (B) Fluorine is the best oxidizing agent. (C) Fluorine atoms have the smallest radii. (D) Iodine ...
... 7. All of the following statements concerning the characteristics of the halogens are true EXCEPT: (A) The first ionization energies (potentials) decrease as the atomic numbers of the halogens increase. (B) Fluorine is the best oxidizing agent. (C) Fluorine atoms have the smallest radii. (D) Iodine ...
CP Chemistry Final Exam Review Sheet
... 50. What is the octet rule? The octet rule states that atoms will gain, lose, or share electrons in order to get a full octet (8 e-) in the valence (outermost) shell of an atom. 51. An ion is a particle with an electrical charge created by the transfer (loss or gaining) of electrons. 52. What is a c ...
... 50. What is the octet rule? The octet rule states that atoms will gain, lose, or share electrons in order to get a full octet (8 e-) in the valence (outermost) shell of an atom. 51. An ion is a particle with an electrical charge created by the transfer (loss or gaining) of electrons. 52. What is a c ...
Chapter 9: Electrons in Atoms
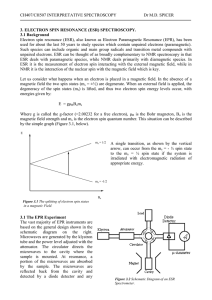
... Electromagnetic radiation is a form of energy transition in which electric and magnetic fields are propagated as waves through empty space (a vacuum) or through a medium such as glass. An electric field is the region around as electrically charged particle. A magnetic field is found in the region su ...
... Electromagnetic radiation is a form of energy transition in which electric and magnetic fields are propagated as waves through empty space (a vacuum) or through a medium such as glass. An electric field is the region around as electrically charged particle. A magnetic field is found in the region su ...
Document
... It is important for you to come to class prepared, i.e. be familiar with the material to be presented. To test your preparedness, a simple five-minute quiz, testing your qualitative familiarity with the material to be discussed in class, will be given at the beginning of some of the classes. No make ...
... It is important for you to come to class prepared, i.e. be familiar with the material to be presented. To test your preparedness, a simple five-minute quiz, testing your qualitative familiarity with the material to be discussed in class, will be given at the beginning of some of the classes. No make ...
S1 Synthesis of Graphene Aerogel with High Electrical Conductivity
... Corresponding author Current address: Department of Materials Science & Engineering, University of Washington, Seattle, WA 98195-2120 ...
... Corresponding author Current address: Department of Materials Science & Engineering, University of Washington, Seattle, WA 98195-2120 ...
Review for Test II
... A. Positive and negative charges (Thompson)-plum pudding model B. Mostly empty space (Rutherford) C. Current nuclear theory of the atom 1. Most of the atom's mass and all of its positive charge is contained in the nucleus 2. Most of the atom is empty space with small fast moving electrons 3. Number ...
... A. Positive and negative charges (Thompson)-plum pudding model B. Mostly empty space (Rutherford) C. Current nuclear theory of the atom 1. Most of the atom's mass and all of its positive charge is contained in the nucleus 2. Most of the atom is empty space with small fast moving electrons 3. Number ...
Part 1
... final ninitial where RH = 2.178 x 10-18 J, Z is the atomic number of the element, and the n-values are the quantum numbers for the initial (higher n value) and final (lower n value) energy levels of an ...
... final ninitial where RH = 2.178 x 10-18 J, Z is the atomic number of the element, and the n-values are the quantum numbers for the initial (higher n value) and final (lower n value) energy levels of an ...
Section 7: Free electron model
... freely mobile, then the electronic contribution to the heat capacity should be 3/2NkB, just as for the atoms of a monatomic gas. But the observed electronic contribution at room temperature is usually less than 0.01 of this value. This discrepancy was resolved only upon the discovery of the Pauli ex ...
... freely mobile, then the electronic contribution to the heat capacity should be 3/2NkB, just as for the atoms of a monatomic gas. But the observed electronic contribution at room temperature is usually less than 0.01 of this value. This discrepancy was resolved only upon the discovery of the Pauli ex ...
Quantum - LearningHood
... • Describes the spin in either of two possible directions. Each orbital can be filled by only two electrons, each with an opposite spin. ...
... • Describes the spin in either of two possible directions. Each orbital can be filled by only two electrons, each with an opposite spin. ...
Electron Distribution Using Peas
... Knowledge of the behavior of electrons in the atom comes from theoretical work done in the 1920s by Heisenberg and Schrodinger. Heisenberg postulated that it was impossible to determine exactly both the position and momentum of an electron at the same instant. Heisenberg deduced that the more precis ...
... Knowledge of the behavior of electrons in the atom comes from theoretical work done in the 1920s by Heisenberg and Schrodinger. Heisenberg postulated that it was impossible to determine exactly both the position and momentum of an electron at the same instant. Heisenberg deduced that the more precis ...
Wave-particle_duality
... An α-particle having a de Broglie wavelength λi collides with a stationary carbon nucleus. The α-particle moves off in a different direction as shown below. final direction of –particle, de Broglie wavelength f ...
... An α-particle having a de Broglie wavelength λi collides with a stationary carbon nucleus. The α-particle moves off in a different direction as shown below. final direction of –particle, de Broglie wavelength f ...
Section 3.6
... quantum number might be n = 2, l = 1, ml = –1, and ms = +1/2 . This might describe an electron in a hydrogen atom in an “excited” state. 7. For each principal quantum number from n = 1 to n = 3 (see Table 4), there can be 2, 8, and 18 different electron descriptions. 8. In the development of scienti ...
... quantum number might be n = 2, l = 1, ml = –1, and ms = +1/2 . This might describe an electron in a hydrogen atom in an “excited” state. 7. For each principal quantum number from n = 1 to n = 3 (see Table 4), there can be 2, 8, and 18 different electron descriptions. 8. In the development of scienti ...
Chapter 11
... heating a gas or with electricity we can get it to give off colors. Passing this light through a prism does something different. ...
... heating a gas or with electricity we can get it to give off colors. Passing this light through a prism does something different. ...
Lectures 1-2: Introduction to Atomic Spectroscopy Types of Spectra
... Substituting in for constants, Eqn. 3 can be written and Eqn. 2 can be written ...
... Substituting in for constants, Eqn. 3 can be written and Eqn. 2 can be written ...
PHY583 - Note 1e - Free Electron Theory of Metal
... 1. the random thermal displacements (thermal vibrations) of ions about lattice points 2. other deviations from a perfect lattice such as impurity atoms & defects that scatter electron ...
... 1. the random thermal displacements (thermal vibrations) of ions about lattice points 2. other deviations from a perfect lattice such as impurity atoms & defects that scatter electron ...
POWERPOINT JEOPARDY
... How many electrons are needed to fill the first, second, and third principal energy levels in the Bohr Model of the atom? Draw the Bohr Models for Aluminum and Neon. What is an “orbital” (in the Quantum Mechanical Model)? How is it different than an “orbit” (in the Bohr Model)? How does the ...
... How many electrons are needed to fill the first, second, and third principal energy levels in the Bohr Model of the atom? Draw the Bohr Models for Aluminum and Neon. What is an “orbital” (in the Quantum Mechanical Model)? How is it different than an “orbit” (in the Bohr Model)? How does the ...
Chapter 4 Review
... 14. Why can’t photons be used to find the location of electrons? (ANS: photons of light used as a probe move the electron as its measuring its location) 15. How do we know what elements are in Jupiter’s atmosphere if we have never been there? (ANS: The elements in Jupiter emits light. When the light ...
... 14. Why can’t photons be used to find the location of electrons? (ANS: photons of light used as a probe move the electron as its measuring its location) 15. How do we know what elements are in Jupiter’s atmosphere if we have never been there? (ANS: The elements in Jupiter emits light. When the light ...
THE PHOTOELECTRIC EFFECT
... The phototube uses an emitter made of potassium metal. The accepted value for the work function of potassium is 2.24 eV, but there are other sources of voltage in the experiment, such as contact potentials of dissimilar metals, that may distort this value. The collector is a circular wire constructe ...
... The phototube uses an emitter made of potassium metal. The accepted value for the work function of potassium is 2.24 eV, but there are other sources of voltage in the experiment, such as contact potentials of dissimilar metals, that may distort this value. The collector is a circular wire constructe ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.