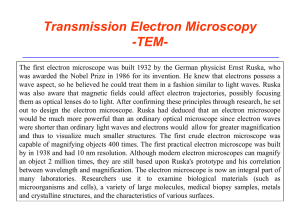
2.8 Atomic Spectra of Hydrogen For some time scientist had known
... 2.8 Atomic Spectra of Hydrogen For some time scientist had known that every atom, when subjected to high temperatures or an electrical discharge, emits electromagnetic radiation of characteristic frequencies or each atom has a characteristic emission spectrum. Because the emission spectra of atoms c ...
... 2.8 Atomic Spectra of Hydrogen For some time scientist had known that every atom, when subjected to high temperatures or an electrical discharge, emits electromagnetic radiation of characteristic frequencies or each atom has a characteristic emission spectrum. Because the emission spectra of atoms c ...
Chem fundamentals 1a
... about the GC and GE principles as tools to reach sustainable societies globally, what would be your wish list for the ideal chemical pesticide? What would be its properties and what would you specify about how it is made, used, and disposed of? • Related question: What is the distinction between ...
... about the GC and GE principles as tools to reach sustainable societies globally, what would be your wish list for the ideal chemical pesticide? What would be its properties and what would you specify about how it is made, used, and disposed of? • Related question: What is the distinction between ...
Atoms and quantum phenomena
... • Whilst we are at it, small amounts of energy mean that the Joule is a bit cumbersome. So we use the Electron Volt • Definition: The amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt. (Not SI, experimental) • 1eV = ...
... • Whilst we are at it, small amounts of energy mean that the Joule is a bit cumbersome. So we use the Electron Volt • Definition: The amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt. (Not SI, experimental) • 1eV = ...
Chapter 6 Electronic Structure of Atoms
... Introduced the Wave Theory of Matter where he proposed that all moving particles, particularly subatomic particles such as electrons, exhibit a degree of wave-like behavior. ...
... Introduced the Wave Theory of Matter where he proposed that all moving particles, particularly subatomic particles such as electrons, exhibit a degree of wave-like behavior. ...
1 Spring 2008 Chemistry 1000 Midterm #1B
... The wavelength of the light needed to excited an electron in a hydrogen atom from the 4s to the 6 p orbital is the same as that necessary for the excitation from the 4s to the 6s orbital, since the 6s and 6p orbitals have the same energy in the hydrogen atom. The energy is only different for multiel ...
... The wavelength of the light needed to excited an electron in a hydrogen atom from the 4s to the 6 p orbital is the same as that necessary for the excitation from the 4s to the 6s orbital, since the 6s and 6p orbitals have the same energy in the hydrogen atom. The energy is only different for multiel ...
Notes on the Electronic Structure of Atoms
... • An orbital is described by a set of three quantum An orbital is described by a set of three quantum numbers. • Quantum numbers can be considered to be Quantum numbers can be considered to be “coordinates” (similar to x, y, and z coodrinates g p ) which are related to where an for a graph) elec ...
... • An orbital is described by a set of three quantum An orbital is described by a set of three quantum numbers. • Quantum numbers can be considered to be Quantum numbers can be considered to be “coordinates” (similar to x, y, and z coodrinates g p ) which are related to where an for a graph) elec ...
Microsoft Word Format - University of Toronto Physics
... Figure 2. Lowest order Feynman graph for Compton scattering from a free electron. The graph is one-dimensional, the solid line representing the electron moving forward in time, and the wavy paths representing the incident and emitted photons. Because of the uncertainty principle energy and momentum ...
... Figure 2. Lowest order Feynman graph for Compton scattering from a free electron. The graph is one-dimensional, the solid line representing the electron moving forward in time, and the wavy paths representing the incident and emitted photons. Because of the uncertainty principle energy and momentum ...
Chemistry 1000 (Fall 2011) Problem Set #2: Orbitals and Electrons
... element 47 should therefore release more heat than adding a second electron into one of the three (partially filled) 4p orbitals in Morspin element 48. This is because there will be enough electrostatic repulsion between the two electrons in the same p-orbital to counteract the fact that Morspin ele ...
... element 47 should therefore release more heat than adding a second electron into one of the three (partially filled) 4p orbitals in Morspin element 48. This is because there will be enough electrostatic repulsion between the two electrons in the same p-orbital to counteract the fact that Morspin ele ...
Chemistry 106: General Chemistry
... I. Sodium (Na), has a larger atomic radius than Cesium (Cs). II. The first ionization energy of an atom generally increases moving left to right across a period because the effective nuclear charge, Zeff, increases in that direction. III. An atom of Phosphorus (P), releases more energy (becomes more ...
... I. Sodium (Na), has a larger atomic radius than Cesium (Cs). II. The first ionization energy of an atom generally increases moving left to right across a period because the effective nuclear charge, Zeff, increases in that direction. III. An atom of Phosphorus (P), releases more energy (becomes more ...
Lecture 9 - ChemWeb (UCC)
... 1st excited state: … a2u 2 e1g 3 e2u 1 1 unpaired electron in e1g and 1 in e2u e1g x e2u = B2u + B1u + E1u (check by multiplying to get reducible representation and then reducing it) States of these three symmetries are observed in the absorption spectrum of the molecule. A1g B2u at ~ 220 nm (uv) ...
... 1st excited state: … a2u 2 e1g 3 e2u 1 1 unpaired electron in e1g and 1 in e2u e1g x e2u = B2u + B1u + E1u (check by multiplying to get reducible representation and then reducing it) States of these three symmetries are observed in the absorption spectrum of the molecule. A1g B2u at ~ 220 nm (uv) ...
n= n= n=1
... 9. The most prominent feature of the hydrogen spectrum in the visible region is the red Balmer line, coming from the transition n = 3 to n = 2. First of all, determine the wavelength and frequency of this line, according to the Bohr theory. Fine structure splits this line into several closely spaced ...
... 9. The most prominent feature of the hydrogen spectrum in the visible region is the red Balmer line, coming from the transition n = 3 to n = 2. First of all, determine the wavelength and frequency of this line, according to the Bohr theory. Fine structure splits this line into several closely spaced ...
Chapters 7, 8, 9 notes - SLCUSD Staff Directory
... expected pattern. They are found in the d and f sublevels. For instance, 24Cr actual electron configuration is: [Ar]4s13d5. The actual electron configuration for 29Cu is [Ar]4s13d10. Why does this happen? Because the difference in ______________ between the 4s and 3d is small, the expected 4s2 elect ...
... expected pattern. They are found in the d and f sublevels. For instance, 24Cr actual electron configuration is: [Ar]4s13d5. The actual electron configuration for 29Cu is [Ar]4s13d10. Why does this happen? Because the difference in ______________ between the 4s and 3d is small, the expected 4s2 elect ...
LEP 5.1.08 Atomic spectra of two-electron systems: He, Hg
... In the present case, the orbital angular momentum of the single electron l is equal to the total angular momentum of the two electrons L, since only one-particle excitations are being considered and the second electron remains in the ground state (l = 0). Cnl and Anl are the coulomb and exchange ene ...
... In the present case, the orbital angular momentum of the single electron l is equal to the total angular momentum of the two electrons L, since only one-particle excitations are being considered and the second electron remains in the ground state (l = 0). Cnl and Anl are the coulomb and exchange ene ...
pptx
... A brief review of chemistry Electron configuration in atoms: How do the electrons fit into the available orbitals? What are energies of orbitals? 1, 2, 3 … principle quantum number, tells you some about energy s, p, d … tells you some about geometric configuration of orbital 3d ...
... A brief review of chemistry Electron configuration in atoms: How do the electrons fit into the available orbitals? What are energies of orbitals? 1, 2, 3 … principle quantum number, tells you some about energy s, p, d … tells you some about geometric configuration of orbital 3d ...
Topic 1 Test - A-Level Chemistry
... Write an equation, including state symbols, to show the reaction that occurs when the first ionisation energy of Kr is measured. Sometimes the mass spectrum of Kr has a very small peak with an m/z value of 42. Explain the occurrence of this peak. ...
... Write an equation, including state symbols, to show the reaction that occurs when the first ionisation energy of Kr is measured. Sometimes the mass spectrum of Kr has a very small peak with an m/z value of 42. Explain the occurrence of this peak. ...
Electrophilic Additions to Double Bonds
... each spatial orbital can be combined with an alpha or beta spin component to form a spin orbital ...
... each spatial orbital can be combined with an alpha or beta spin component to form a spin orbital ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.