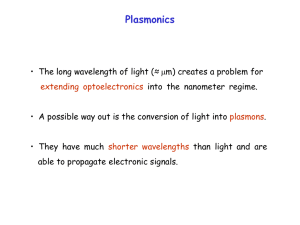
Electronic structure of correlated electron systems
... the electron charge density in DFT would also correspond to exactly one electron per atom but the wave function would be a single Slater determinant of one electron Bloch waves and not a single Slater determinant of atomic site localized s orbitals with one electron at each site. In the DFT case the ...
... the electron charge density in DFT would also correspond to exactly one electron per atom but the wave function would be a single Slater determinant of one electron Bloch waves and not a single Slater determinant of atomic site localized s orbitals with one electron at each site. In the DFT case the ...
Rdg: Electron Configuration
... The number of sublevels that an energy level can contain is equal to the principle quantum number of that level. So, for example, the second energy level would have two sublevels, and the third energy level would have three sublevels. The first sublevel is called an s sublevel. The second sublevel i ...
... The number of sublevels that an energy level can contain is equal to the principle quantum number of that level. So, for example, the second energy level would have two sublevels, and the third energy level would have three sublevels. The first sublevel is called an s sublevel. The second sublevel i ...
Ideas to Implementation by Jonathan Chan
... However, a major problem is that when used in electronic components, it gets hot and becomes a relatively good conductor, allowing too much current to pass through and possibly damage electronic equipment. The issue was that the resistance to electric current flow that makes the semiconductor useful ...
... However, a major problem is that when used in electronic components, it gets hot and becomes a relatively good conductor, allowing too much current to pass through and possibly damage electronic equipment. The issue was that the resistance to electric current flow that makes the semiconductor useful ...
Coulomb Drag to Measure Electron-Electron Interaction in Bilayer
... Notice that individual layer scattering times are going to disappear from the ratio between E1 and I2. This is immensely important - because we have now related a transport measurement to electron-electron scattering . The effect of disorder has somehow disappeared - at least within the relaxation t ...
... Notice that individual layer scattering times are going to disappear from the ratio between E1 and I2. This is immensely important - because we have now related a transport measurement to electron-electron scattering . The effect of disorder has somehow disappeared - at least within the relaxation t ...
(TEQ) Model of the Electron - Superluminal quantum models of the
... • is a helically moving point-like quantum object having a frequency and a wavelength, and carrying energy and momentum. • can easily pass through the speed of light (being massless). • can generate a photon or an electron depending on whether the energy quantum’s helical trajectory is open or close ...
... • is a helically moving point-like quantum object having a frequency and a wavelength, and carrying energy and momentum. • can easily pass through the speed of light (being massless). • can generate a photon or an electron depending on whether the energy quantum’s helical trajectory is open or close ...
Today: Bohr Model - University of Colorado Boulder
... Bohr hypothesis: only certain E levels. e hop down to lowest level, giving off photons when make jump, stable in lowest level. ...
... Bohr hypothesis: only certain E levels. e hop down to lowest level, giving off photons when make jump, stable in lowest level. ...
Electron Configuration of Atoms
... Periodicity of Electron Configurations • The principal energy level number, the number that comes before the sublevel letter designation, is the same as the period number for the s and p sublevels. • For the d sublevels, the principal energy level number is one less than the period number. Why? ...
... Periodicity of Electron Configurations • The principal energy level number, the number that comes before the sublevel letter designation, is the same as the period number for the s and p sublevels. • For the d sublevels, the principal energy level number is one less than the period number. Why? ...
Untitled
... • Atoms are bonded covalently into 2-D sheets that are held together with van der Waals forces. – They tend to be softer and have lower melting points then 3-D network solids because the 2-D layers can slide past each other relatively easily ...
... • Atoms are bonded covalently into 2-D sheets that are held together with van der Waals forces. – They tend to be softer and have lower melting points then 3-D network solids because the 2-D layers can slide past each other relatively easily ...
Electron-beam lithography

Electron-beam lithography (often abbreviated as e-beam lithography) is the practice of scanning a focused beam of electrons to draw custom shapes on a surface covered with an electron-sensitive film called a resist (""exposing""). The electron beam changes the solubility of the resist, enabling selective removal of either the exposed or non-exposed regions of the resist by immersing it in a solvent (""developing""). The purpose, as with photolithography, is to create very small structures in the resist that can subsequently be transferred to the substrate material, often by etching.The primary advantage of electron-beam lithography is that it can draw custom patterns (direct-write) with sub-10 nm resolution. This form of maskless lithography has high resolution and low throughput, limiting its usage to photomask fabrication, low-volume production of semiconductor devices, and research & development.