Chapter 4 – Reactions in Aqueous Solutions
... – Note: the above equation is both not balanced and not complete. It only shows the components (reactants) that undergoes changes is oxidation numbers; – Redox reactions in acidic solution means that you need to add H+ ion in the equation, which produces water as one of the products. ...
... – Note: the above equation is both not balanced and not complete. It only shows the components (reactants) that undergoes changes is oxidation numbers; – Redox reactions in acidic solution means that you need to add H+ ion in the equation, which produces water as one of the products. ...
New Title
... Use Target Reading Skills After you read the section, reread the paragraphs that contain definitions of Key Terms. Use all of the information you have learned to write a meaningful sentence using each Key Term. a. chemical equation: ____________________________________________________ ...
... Use Target Reading Skills After you read the section, reread the paragraphs that contain definitions of Key Terms. Use all of the information you have learned to write a meaningful sentence using each Key Term. a. chemical equation: ____________________________________________________ ...
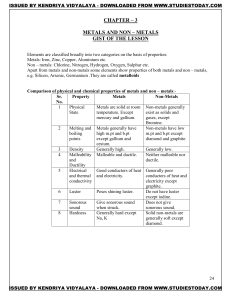
METALS AND NON – METALS Concepts
... + Cl2 2NaCl Metals react with hydrogen to form metal hydride This reaction takes place only for most reactive metals. 2Na(s) + H2(g) 2NaH(s) ...
... + Cl2 2NaCl Metals react with hydrogen to form metal hydride This reaction takes place only for most reactive metals. 2Na(s) + H2(g) 2NaH(s) ...
GENERAL CHEMISTRY REVIEW
... concentration of reactants and products in terms of x, the extent of reaction. Substitute these equilibrium concentrations into the expression for the equilibrium constant and solve for x. Once x is known it can be substituted into the earlier equations to give the equilibrium concentrations. These ...
... concentration of reactants and products in terms of x, the extent of reaction. Substitute these equilibrium concentrations into the expression for the equilibrium constant and solve for x. Once x is known it can be substituted into the earlier equations to give the equilibrium concentrations. These ...
Chemistry - Edgbarrow School
... I can describe dissolving, thermal decomposition, oxidation, of reactivity for Group 1 and human activities impact on may be absorbed or different materials based on with reference to particles displacement and the reaction of Group 7 in the Periodic the climate by producing released during the maki ...
... I can describe dissolving, thermal decomposition, oxidation, of reactivity for Group 1 and human activities impact on may be absorbed or different materials based on with reference to particles displacement and the reaction of Group 7 in the Periodic the climate by producing released during the maki ...
chm 434f/1206f solid state materials chemistry
... SHAPE, SIZE AND DEFECTS ARE EVERYTHING! • Form or morphology and physical size of product controls synthesis method of choice and potential utility • Single crystal, phase pure, defect free solids - do not exist and if they did not likely of much interest! • Single crystal (SC) that has been defect ...
... SHAPE, SIZE AND DEFECTS ARE EVERYTHING! • Form or morphology and physical size of product controls synthesis method of choice and potential utility • Single crystal, phase pure, defect free solids - do not exist and if they did not likely of much interest! • Single crystal (SC) that has been defect ...
EXAM 3
... The elements nitrogen and oxygen combine at high temperatures to form nitric oxide, NO. The balanced chemical equation is N2(g) + O2(g) ----------> 2NO(g) In a high temperature experiment, a chemist mixed 3.417 g of N2 with an excess of O2 and allowed the above reaction to take place. Assuming compl ...
... The elements nitrogen and oxygen combine at high temperatures to form nitric oxide, NO. The balanced chemical equation is N2(g) + O2(g) ----------> 2NO(g) In a high temperature experiment, a chemist mixed 3.417 g of N2 with an excess of O2 and allowed the above reaction to take place. Assuming compl ...
Chemistry II Aqueous Reactions and Solution Chemistry Chapter 4
... are substances that ionize in aqueous solutions to form hydrogen ions, increasing the concentration of hydrogen ions in solution. Because hydrogen ions are just a proton, acids are known as proton ...
... are substances that ionize in aqueous solutions to form hydrogen ions, increasing the concentration of hydrogen ions in solution. Because hydrogen ions are just a proton, acids are known as proton ...
H2-rich fluids from serpentinization: Geochemical and biotic
... buffer metamorphic fluids to extremely reducing conditions that are capable of producing hydrogen gas. Awaruite, FeNi3, forms early in this process when the serpentinite minerals are Fe-rich. Olivine with the current mantle Fe兾Mg ratio was oxidized during serpentinization after the Moon-forming impa ...
... buffer metamorphic fluids to extremely reducing conditions that are capable of producing hydrogen gas. Awaruite, FeNi3, forms early in this process when the serpentinite minerals are Fe-rich. Olivine with the current mantle Fe兾Mg ratio was oxidized during serpentinization after the Moon-forming impa ...
8.5DF: Chemical Formulas and Equations
... Cooking with Chemical Formulas and Equations To help students learn more about chemical formulas and equations, work with your child to explain how equations are similar to a recipe that might be used while cooking. Interestingly, there are many different ways that chemical reactions and chemical eq ...
... Cooking with Chemical Formulas and Equations To help students learn more about chemical formulas and equations, work with your child to explain how equations are similar to a recipe that might be used while cooking. Interestingly, there are many different ways that chemical reactions and chemical eq ...
Chemistry 20 Lesson 36 – The Whole Enchilada
... ___ Cu(s) + ___ HNO3 (aq) → ___Cu(NO3)2 (aq) + ___ NO(g) + ___ H2O(1) ...
... ___ Cu(s) + ___ HNO3 (aq) → ___Cu(NO3)2 (aq) + ___ NO(g) + ___ H2O(1) ...
Chapter 12 Oxidation-Reduction Reactions
... • The oxidation number of hydrogen is +1 on both sides of the equation, so hydrogen is neither oxidized nor reduced. • The oxidation number of oxygen is −2 on both sides of the equation, so oxygen is neither oxidized nor reduced. • The only remaining atoms are nitrogen atoms. Nitrogen atoms are foun ...
... • The oxidation number of hydrogen is +1 on both sides of the equation, so hydrogen is neither oxidized nor reduced. • The oxidation number of oxygen is −2 on both sides of the equation, so oxygen is neither oxidized nor reduced. • The only remaining atoms are nitrogen atoms. Nitrogen atoms are foun ...
Copper Coordination Polymers with Infinite Chloride Ion Channels
... value for Cu2+ is 1.73 pB). Antiparallel alignment by antiferromagnetic coupling in a through-bond (Cu-OH-Cu or Cu-triazolyl-Cu[”J) interaction can result in a smaller effective magnetic moment which further decreases when the temperature is lowered as can be seen in Figure 5. Because of its two-dim ...
... value for Cu2+ is 1.73 pB). Antiparallel alignment by antiferromagnetic coupling in a through-bond (Cu-OH-Cu or Cu-triazolyl-Cu[”J) interaction can result in a smaller effective magnetic moment which further decreases when the temperature is lowered as can be seen in Figure 5. Because of its two-dim ...
Chapter 6: Chemical Equilibrium
... 9. The reaction, Q + 2 SO3(g) 2 SO2(g) + O2(g) is endothermic. Predict what will happen if the temperature is increased. a. Kc remains the same b. Kc decreases c. the pressure decreases d. more SO3(g) is produced * e. Kc increases T increase, reaction will shift to right side and Kc increase 10. Con ...
... 9. The reaction, Q + 2 SO3(g) 2 SO2(g) + O2(g) is endothermic. Predict what will happen if the temperature is increased. a. Kc remains the same b. Kc decreases c. the pressure decreases d. more SO3(g) is produced * e. Kc increases T increase, reaction will shift to right side and Kc increase 10. Con ...
BERKELEY HEIGHTS PUBLIC SCHOOLS
... Students are made aware of how mathematics is a valuable tool for scientific inquiry. In conjunction with learning the International Union of Pure and Applied Chemists (IUPAC) system of nomenclature, students can appreciate the ease and rationale for using these approaches. With this background, stu ...
... Students are made aware of how mathematics is a valuable tool for scientific inquiry. In conjunction with learning the International Union of Pure and Applied Chemists (IUPAC) system of nomenclature, students can appreciate the ease and rationale for using these approaches. With this background, stu ...
Student Exploration Sheet: Growing Plants
... substances can combine during a chemical reaction to produce new substances. The substances that undergo change are called reactants. The new substances are products. Sometimes during a chemical reaction, one type of reactant will be used up before the other reactants. This reactant is the limiting ...
... substances can combine during a chemical reaction to produce new substances. The substances that undergo change are called reactants. The new substances are products. Sometimes during a chemical reaction, one type of reactant will be used up before the other reactants. This reactant is the limiting ...
Summer Assignment
... 5. Oxygen has an oxidation number of –2 unless it is combined with F, when it is +2, or it is in a peroxide, when it is –1. ...
... 5. Oxygen has an oxidation number of –2 unless it is combined with F, when it is +2, or it is in a peroxide, when it is –1. ...
Standard Voltages Cell Voltage
... standard voltages for reduction half reactions • Standard voltages for oxidation half reactions are obtained by reversing these reactions and changing the sign of the Eored value ...
... standard voltages for reduction half reactions • Standard voltages for oxidation half reactions are obtained by reversing these reactions and changing the sign of the Eored value ...
Characteristics of Emissions from Municipal Waste Landfills
... 4. Hydrogen sulphide formation Due to the hydrogen sulphide, landfill gases have a peculiar odour of rotten eggs. The unpleasant odour is perceptible even at very low concentrations. Some people with a very low odour perception level can detect sulphide at concentrations as low as 0.5 ppb (parts per ...
... 4. Hydrogen sulphide formation Due to the hydrogen sulphide, landfill gases have a peculiar odour of rotten eggs. The unpleasant odour is perceptible even at very low concentrations. Some people with a very low odour perception level can detect sulphide at concentrations as low as 0.5 ppb (parts per ...
Chapter 4
... added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the ...
... added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the ...
① Name AP CHEM __/__/__ Chapter 12 Outline
... exist only because our bodies contain many substances called enzymes, which increase the rates of these reactions. Almost every biologically important reaction is assisted by a specific enzyme. A catalyst is a substance that speeds up a chemical reaction without being consumed itself. Almost all i ...
... exist only because our bodies contain many substances called enzymes, which increase the rates of these reactions. Almost every biologically important reaction is assisted by a specific enzyme. A catalyst is a substance that speeds up a chemical reaction without being consumed itself. Almost all i ...
Redox Reactions C12-1-10
... Remember that although redox reactions are common, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to another. In this redox reaction between sodium and iodine: 2Na + I2 -->2NaI electrons are completely transferred f ...
... Remember that although redox reactions are common, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to another. In this redox reaction between sodium and iodine: 2Na + I2 -->2NaI electrons are completely transferred f ...
February 13, 2008
... A. At equilibrium, the total concentration of products equals the total concentration of reactants B. Equilibrium is the result of the cessation of all chemical change. C. There is only one set of equilibrium concentrations that equals the Kc value. D. The rate constant of the forward reaction is eq ...
... A. At equilibrium, the total concentration of products equals the total concentration of reactants B. Equilibrium is the result of the cessation of all chemical change. C. There is only one set of equilibrium concentrations that equals the Kc value. D. The rate constant of the forward reaction is eq ...