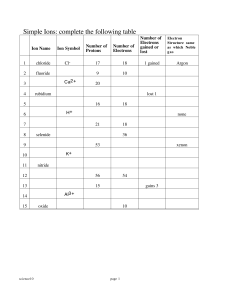
Part One: Ions in Aqueous Solution A. Electrolytes and Non
... HAc(aq) + NaOH(aq) → NaAc(aq) + H2O(l) Total ionic reaction: HAc(aq) + [Na+(aq) + OH (aq)] → [Na+(aq) + Ac (aq)] + H2O(l) Net ionic reaction for weak acid + strong base: HAc(aq) + OH-(aq) → Ac-(aq) + H2O(l) ...
... HAc(aq) + NaOH(aq) → NaAc(aq) + H2O(l) Total ionic reaction: HAc(aq) + [Na+(aq) + OH (aq)] → [Na+(aq) + Ac (aq)] + H2O(l) Net ionic reaction for weak acid + strong base: HAc(aq) + OH-(aq) → Ac-(aq) + H2O(l) ...
CHEMISTRY SEC 06 SYLLABUS
... The coursework should not be restricted to an acquisition of information but should assess students' ability to: select procedures, plan and organize experimental investigations to test a hypothesis, validateconclusions or solve a chemical problem; organize data and perform calculations in which gui ...
... The coursework should not be restricted to an acquisition of information but should assess students' ability to: select procedures, plan and organize experimental investigations to test a hypothesis, validateconclusions or solve a chemical problem; organize data and perform calculations in which gui ...
CHEMISTRY SEC 06 SYLLABUS
... The coursework should not be restricted to an acquisition of information but should assess students' ability to: select procedures, plan and organize experimental investigations to test a hypothesis, validateconclusions or solve a chemical problem; organize data and perform calculations in which gui ...
... The coursework should not be restricted to an acquisition of information but should assess students' ability to: select procedures, plan and organize experimental investigations to test a hypothesis, validateconclusions or solve a chemical problem; organize data and perform calculations in which gui ...
CHEMISTRY SEC 06 SYLLABUS
... The coursework should not be restricted to an acquisition of information but should assess students' ability to: select procedures, plan and organize experimental investigations to test a hypothesis, validateconclusions or solve a chemical problem; organize data and perform calculations in which gui ...
... The coursework should not be restricted to an acquisition of information but should assess students' ability to: select procedures, plan and organize experimental investigations to test a hypothesis, validateconclusions or solve a chemical problem; organize data and perform calculations in which gui ...
REACTING MASSES – ACTIVITY SHEET
... ammonia with an excess of sodium chlorate. b) In the reaction, only 280 g of hydrazine was produced. Calculate the percentage yield. c) Calculate the atom economy for this way of making hydrazine. 2) Ibuprofen is a common pain killer used for symptoms such as head aches, tooth ache and period pains. ...
... ammonia with an excess of sodium chlorate. b) In the reaction, only 280 g of hydrazine was produced. Calculate the percentage yield. c) Calculate the atom economy for this way of making hydrazine. 2) Ibuprofen is a common pain killer used for symptoms such as head aches, tooth ache and period pains. ...
chem equation Pkt Student2
... 3) Write a balanced chemical equation by adding_____________________, NOT subscripts (this will require trial and error, the following guidelines may be helpful) a) balance the different types of atoms ________________ b) first, balance the atoms of elements that are combined and that appear only _ ...
... 3) Write a balanced chemical equation by adding_____________________, NOT subscripts (this will require trial and error, the following guidelines may be helpful) a) balance the different types of atoms ________________ b) first, balance the atoms of elements that are combined and that appear only _ ...
LIQUIDS
... After element 20 the electron arrangement becomes more complicated, but it is always true that elements in Group 1 have one electron in their outer shell, so we can say that Rb, Cs and Fr will all have one electron in their outer shell. Therefore elements in Group 3 always have three electrons in th ...
... After element 20 the electron arrangement becomes more complicated, but it is always true that elements in Group 1 have one electron in their outer shell, so we can say that Rb, Cs and Fr will all have one electron in their outer shell. Therefore elements in Group 3 always have three electrons in th ...
workbook Chem (WP)
... 1. identify the theories and their authors represented by the following: a. atom looks like this b. atom looks like a planetary system c. the electrons move around the nucleus in an unknown path. d. key was the “Gold foil experiment”. Ions 1. Why do ions form? 2. Draw the Bohr diagram for the atom a ...
... 1. identify the theories and their authors represented by the following: a. atom looks like this b. atom looks like a planetary system c. the electrons move around the nucleus in an unknown path. d. key was the “Gold foil experiment”. Ions 1. Why do ions form? 2. Draw the Bohr diagram for the atom a ...
Topic 7b Redox notes
... hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
... hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
examination review
... YOU WILL LEARN A LOT MORE ABOUT WEAK ACIDS LATER IN THIS UNIT! It is worth remembering at this time that acidic solutions are also created by certain substances that react with water to form H+(aq)ions. You have already learned that non-metal oxides react with water to form acidic solutions. For exa ...
... YOU WILL LEARN A LOT MORE ABOUT WEAK ACIDS LATER IN THIS UNIT! It is worth remembering at this time that acidic solutions are also created by certain substances that react with water to form H+(aq)ions. You have already learned that non-metal oxides react with water to form acidic solutions. For exa ...
Section 4.8: Acid-Base Reactions
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions in a precipitation reaction will keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions in a precipitation reaction will keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
C:\usb key\sch3u\unit 1\chapter 2 test answers.wpd
... There are no co-ordinate covalent bonds. 7) Give the Lewis structure, compound type (ionic, polar, non-polar), shape and shape code (if molecular), for each of the following. a) CH4 b) KI c) CH2F2 d) SO3 e) N2 non-polar ionic polar non-polar non-polar ...
... There are no co-ordinate covalent bonds. 7) Give the Lewis structure, compound type (ionic, polar, non-polar), shape and shape code (if molecular), for each of the following. a) CH4 b) KI c) CH2F2 d) SO3 e) N2 non-polar ionic polar non-polar non-polar ...
ch15-Atmospheric Chemistry
... (iv) Photoionization (formation of ions in the ionsphere) N2* N2+ + e- ...
... (iv) Photoionization (formation of ions in the ionsphere) N2* N2+ + e- ...
View PDF
... ____ 17. In the reaction A + B → C + D, if the quantity of B is insufficient to react with all of A, a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a che ...
... ____ 17. In the reaction A + B → C + D, if the quantity of B is insufficient to react with all of A, a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a che ...
Chemical Equations - Salem Community Schools
... physical state of each reactant and product. How can we indicate the bubbles we see during this reaction are CO2? Symbols in the parentheses are put after formulas to indicate the state of the substance. Solids, liquids, gases, and water (aqueous) solutions are indicated by the symbols (s), (l), (g) ...
... physical state of each reactant and product. How can we indicate the bubbles we see during this reaction are CO2? Symbols in the parentheses are put after formulas to indicate the state of the substance. Solids, liquids, gases, and water (aqueous) solutions are indicated by the symbols (s), (l), (g) ...
AP Chemistry
... this process assumes reaction takes place in acid (H+), if in base, add an OH- for each H+ in the final equation (combine H+ and OH- to make water) 4. reduction half reactions of common oxidizing agents a. MnO4- + 8 H+ + 5 e- Mn2+ + 4 H2O b. Cr2O72- + 14 H+ + 6 e- 2 Cr3+ + 7 H2O 5. oxidation of ...
... this process assumes reaction takes place in acid (H+), if in base, add an OH- for each H+ in the final equation (combine H+ and OH- to make water) 4. reduction half reactions of common oxidizing agents a. MnO4- + 8 H+ + 5 e- Mn2+ + 4 H2O b. Cr2O72- + 14 H+ + 6 e- 2 Cr3+ + 7 H2O 5. oxidation of ...
Catalyst characterization: characterization techniques
... difficult access like EXAFS, requiring synchrotron radiation, will be reported because of their importance); (iii) applicability to many different systems (then little space will be devoted to Miissbauer and PAS, much more to N, adsorption, IR spectroscopy and temperature programmed techniques, etc. ...
... difficult access like EXAFS, requiring synchrotron radiation, will be reported because of their importance); (iii) applicability to many different systems (then little space will be devoted to Miissbauer and PAS, much more to N, adsorption, IR spectroscopy and temperature programmed techniques, etc. ...
Unit C3 - Chemistry in Action
... we want to analyse this chemical. What tests could we do? There are two main types of analysis: 1) Qualitative – descriptions of what is present You need to use different tests for different ions – for example, if this chemical contains copper chloride then we’d need to verify by testing for copper ...
... we want to analyse this chemical. What tests could we do? There are two main types of analysis: 1) Qualitative – descriptions of what is present You need to use different tests for different ions – for example, if this chemical contains copper chloride then we’d need to verify by testing for copper ...
Chapter 6 - Sites @ Suffolk University
... 6 x 1023 hydrogen atoms to make up a single gram! Chemists have chosen a very simple way to deal with the masses of the elements and their compounds by expressing the atomic masses in grams. The gram atomic mass of hydrogen, for instance, is about one gram, and the gram atomic mass of oxygen is abou ...
... 6 x 1023 hydrogen atoms to make up a single gram! Chemists have chosen a very simple way to deal with the masses of the elements and their compounds by expressing the atomic masses in grams. The gram atomic mass of hydrogen, for instance, is about one gram, and the gram atomic mass of oxygen is abou ...
Exam No. 1
... This element will combine with the phosphate ion (PO43-) to form a compound with the formula: **(a) XPO4 (c) X2PO4 ...
... This element will combine with the phosphate ion (PO43-) to form a compound with the formula: **(a) XPO4 (c) X2PO4 ...