One-Pot Catalytic Conversion of Cellulose and of Woody
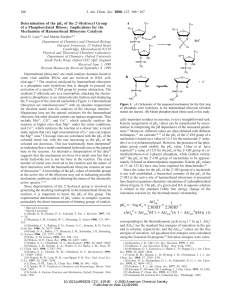
... The reaction time required for solubilization of the biomass solid was probed in a series of experiments where mixtures of pine sawdust (100 mg), Cu20-PMO (100 mg), and MeOH (3.0 mL) were heated at 320 °C in minireactors for the various time intervals: 0.17, 0.33, 0.5, 1.0, 2, 4, 6, 8, 10, and 12 h. ...
... The reaction time required for solubilization of the biomass solid was probed in a series of experiments where mixtures of pine sawdust (100 mg), Cu20-PMO (100 mg), and MeOH (3.0 mL) were heated at 320 °C in minireactors for the various time intervals: 0.17, 0.33, 0.5, 1.0, 2, 4, 6, 8, 10, and 12 h. ...
Chapter 4: Solution Chemistry: The Hydrosphere
... aqueous solution: a solution where water is the dissolving medium (the solvent) – For example, when table salt (NaCl) is dissolved in water, it results in an aqueous solution of sodium chloride, NaCl(aq), with Na+ and Cl- ions dissolved in water. – Note: The physical state aqueous,(aq), indicates an ...
... aqueous solution: a solution where water is the dissolving medium (the solvent) – For example, when table salt (NaCl) is dissolved in water, it results in an aqueous solution of sodium chloride, NaCl(aq), with Na+ and Cl- ions dissolved in water. – Note: The physical state aqueous,(aq), indicates an ...
Chapter 14
... A) all chemical reactions have ceased B) the rates of the forward and reverse reactions are equal C) the rate constants of the forward and reverse reactions are equal D) the value of the equilibrium constant is 1 E) the limiting reagent has been consumed 2) Which one of the following will change the ...
... A) all chemical reactions have ceased B) the rates of the forward and reverse reactions are equal C) the rate constants of the forward and reverse reactions are equal D) the value of the equilibrium constant is 1 E) the limiting reagent has been consumed 2) Which one of the following will change the ...
Section 3_Energetics
... Direct determination of lattice energy is very difficult because it is very difficult to get isolated sodium and chloride ions. Therefore the values are usually calculated from other experimentally determined data by applying the Hess Law. The Born-Haber Cycle is a technique of applying Hess‘s Law t ...
... Direct determination of lattice energy is very difficult because it is very difficult to get isolated sodium and chloride ions. Therefore the values are usually calculated from other experimentally determined data by applying the Hess Law. The Born-Haber Cycle is a technique of applying Hess‘s Law t ...
CHEMISTRY 102 Spring 2012 Hour Exam III Page 20 1. For the
... After equilibrium is reached, which of the following statements (a-d) regarding these experiments is true? a) In experiment 1, the rate of the forward reaction at equilibrium will be greater than the rate of the reverse reaction at equilibrium. b) Equilibrium cannot be reached in experiment 2 becaus ...
... After equilibrium is reached, which of the following statements (a-d) regarding these experiments is true? a) In experiment 1, the rate of the forward reaction at equilibrium will be greater than the rate of the reverse reaction at equilibrium. b) Equilibrium cannot be reached in experiment 2 becaus ...
paper - General Atomics Fusion Group
... transportation fuel that has the potential to displace fossil fuels. Hydrogen will be particularly advantageous when coupled with fuel cells. Fuel cells have higher efficiency than conventional battery/internal combustion engine combinations and do not produce nitrogen oxides during low-temperature ...
... transportation fuel that has the potential to displace fossil fuels. Hydrogen will be particularly advantageous when coupled with fuel cells. Fuel cells have higher efficiency than conventional battery/internal combustion engine combinations and do not produce nitrogen oxides during low-temperature ...
Document
... If 4.0 mol N2 and 1.0 mol O2 are mixed in a 5.0 L container, what are all equilibrium concentrations? ...
... If 4.0 mol N2 and 1.0 mol O2 are mixed in a 5.0 L container, what are all equilibrium concentrations? ...
UNIT 5 - H-W Science Website
... change is that energy is somehow stored in the chemicals and during a transformation there is sometimes excess energy (in which case it will be released) or sometimes not enough energy (in which case it will be absorbed from the surroundings). The change in energy is due mainly to bond rearrangement ...
... change is that energy is somehow stored in the chemicals and during a transformation there is sometimes excess energy (in which case it will be released) or sometimes not enough energy (in which case it will be absorbed from the surroundings). The change in energy is due mainly to bond rearrangement ...
bond enthalpy activity sheet
... ammonia with an excess of sodium chlorate. b) In the reaction, only 280 g of hydrazine was produced. Calculate the percentage yield. c) Calculate the atom economy for this way of making hydrazine. 2) Ibuprofen is a common pain killer used for symptoms such as head aches, tooth ache and period pains. ...
... ammonia with an excess of sodium chlorate. b) In the reaction, only 280 g of hydrazine was produced. Calculate the percentage yield. c) Calculate the atom economy for this way of making hydrazine. 2) Ibuprofen is a common pain killer used for symptoms such as head aches, tooth ache and period pains. ...
Week 7 - Acid-base, redox
... Classifying, Writing, and Balancing Redox Reactions We previously classified, wrote, and balanced precipitation, acid-base, and gas-forming reactions. Redox reactions have electron transfer, and that is what sets them apart from the other reaction types. With redox, one atom loses one or more electr ...
... Classifying, Writing, and Balancing Redox Reactions We previously classified, wrote, and balanced precipitation, acid-base, and gas-forming reactions. Redox reactions have electron transfer, and that is what sets them apart from the other reaction types. With redox, one atom loses one or more electr ...
Hydroxyl Group of a Phosphorylated Ribose
... is a phosphate ester hydrolysis that is thought to proceed via activation of a specific 2′-OH group by proton abstraction. The resultant 2′-alkoxide acts as a nucleophile, attacking the electropositive phosphorus in an intramolecular fashion and displacing the 5′-oxygen of the cleaved nucleotide (Fi ...
... is a phosphate ester hydrolysis that is thought to proceed via activation of a specific 2′-OH group by proton abstraction. The resultant 2′-alkoxide acts as a nucleophile, attacking the electropositive phosphorus in an intramolecular fashion and displacing the 5′-oxygen of the cleaved nucleotide (Fi ...
Unit #8 - consumerchem
... Can anyone tell me what things can be determined by the "coefficient"? Clue: think about the "Limiting ...
... Can anyone tell me what things can be determined by the "coefficient"? Clue: think about the "Limiting ...
Redox Balancing Worksheet
... Fortunately, the film of Ag2S that collects on the metal surface forms a protective coating that slows down further oxidation of the silver metal. For many years, chemists thought of oxidation and reduction as involving the element oxygen in some way or another. That's where the name oxidation came ...
... Fortunately, the film of Ag2S that collects on the metal surface forms a protective coating that slows down further oxidation of the silver metal. For many years, chemists thought of oxidation and reduction as involving the element oxygen in some way or another. That's where the name oxidation came ...
Unit 1 Student Booklet
... Some ions are composed of several atoms joined covalently. These are called polyatomic ions (poly = many). Refer to the Periodic Chart of Polyatomic Ions for a list of ions. The charge for polyatomic ions is for the whole group of atoms not just for the atom written last. DO NOT change the subscript ...
... Some ions are composed of several atoms joined covalently. These are called polyatomic ions (poly = many). Refer to the Periodic Chart of Polyatomic Ions for a list of ions. The charge for polyatomic ions is for the whole group of atoms not just for the atom written last. DO NOT change the subscript ...
Document
... g, at a given temperature is the ideal gas at 1 bar pressure. 2. The standard state of a pure liquid substance, denoted by l, at a given temperature is the pure liquid at 1 bar pressure. 3. The standard state of a pure crystalline substance at a given temperature is the pure crystalline substance, d ...
... g, at a given temperature is the ideal gas at 1 bar pressure. 2. The standard state of a pure liquid substance, denoted by l, at a given temperature is the pure liquid at 1 bar pressure. 3. The standard state of a pure crystalline substance at a given temperature is the pure crystalline substance, d ...
Chemical Equilibrium Stress? What stress? 1
... 2 H2O (l) 2 H2 (g) + O2 (g) The concentration of a pure liquid cannot change, it is fixed and equal to the liquid’s K = [H2]2 [O2] density. [H2O]2 We know that K remains constant for all combinations of reactant and product K[H2O]2 = [H2]2 [O2] = K concentrations at equilibrium. Therefore, ...
... 2 H2O (l) 2 H2 (g) + O2 (g) The concentration of a pure liquid cannot change, it is fixed and equal to the liquid’s K = [H2]2 [O2] density. [H2O]2 We know that K remains constant for all combinations of reactant and product K[H2O]2 = [H2]2 [O2] = K concentrations at equilibrium. Therefore, ...
Chemistry Notes for the Whole Year Powerpoint
... electrons so as to have eight electrons in their outer electron shell. This means that all atoms, in a Lewis structure, must have eight valence electrons around them (they can be either bonded or lone pair electrons). • Hydrogen and helium are exceptions to the octet rule. There is one more element ...
... electrons so as to have eight electrons in their outer electron shell. This means that all atoms, in a Lewis structure, must have eight valence electrons around them (they can be either bonded or lone pair electrons). • Hydrogen and helium are exceptions to the octet rule. There is one more element ...
Experimental and Theoretical Charge Density Analysis of a
... covalent bonds. This molecule has been proved to act as an efficient annulation reagent which results in formation of synthetically challenging and pharmaceutically important 4-, 5-, 6-, and 7-membered heterocycles in excellent yields. The charge density of the molecule was determined from both experi ...
... covalent bonds. This molecule has been proved to act as an efficient annulation reagent which results in formation of synthetically challenging and pharmaceutically important 4-, 5-, 6-, and 7-membered heterocycles in excellent yields. The charge density of the molecule was determined from both experi ...
2009 - NESACS
... 51. Carbon in the Universe comes from 3 He-4 nuclei fusing to create a C-12 atom—A very slow process requiring 100 million K deep inside giant red star core where H is all consumed and He is in abundance. Unstable Be-8 is crucial in creating C-12 but for a split second, 2 He−4 particles fuse to make ...
... 51. Carbon in the Universe comes from 3 He-4 nuclei fusing to create a C-12 atom—A very slow process requiring 100 million K deep inside giant red star core where H is all consumed and He is in abundance. Unstable Be-8 is crucial in creating C-12 but for a split second, 2 He−4 particles fuse to make ...
Chapter 9 Stoichiometry
... When baking cookies, a recipe is usually used, telling the exact amount of each ingredient ...
... When baking cookies, a recipe is usually used, telling the exact amount of each ingredient ...
ch16powerpoint
... The elementary steps must add up to the overall equation. • The elementary steps must be physically reasonable. • The mechanism must correlated with the rate law. ...
... The elementary steps must add up to the overall equation. • The elementary steps must be physically reasonable. • The mechanism must correlated with the rate law. ...
Unit 1
... energy diagrams and energy considerations. Controlling reaction rates is important in many commercial and industrial processes. By applying collision theory to the rates of fast and slow reactions, teachers might look for complete and detailed explanations using the correct terminology. A balloon st ...
... energy diagrams and energy considerations. Controlling reaction rates is important in many commercial and industrial processes. By applying collision theory to the rates of fast and slow reactions, teachers might look for complete and detailed explanations using the correct terminology. A balloon st ...
chemistry (9189)
... bonding; covalent bonding; hydrogen bonding, other intermolecular interactions; metallic bonding) on the physical properties of substances ...
... bonding; covalent bonding; hydrogen bonding, other intermolecular interactions; metallic bonding) on the physical properties of substances ...