Topic 9 - Anderson High School
... These examples are all displacement reactions, because they involve a more reactive metal or non-metal displacing the reactive one from its salt. ...
... These examples are all displacement reactions, because they involve a more reactive metal or non-metal displacing the reactive one from its salt. ...
Energy and Chemistry
... Having to carry its own fuel puts a lot of mass burden on an engine in space. This is why NASA is developing other types of engines to minimize fuel mass. An ion thruster uses xenon atoms that have had at least one electron removed from their atoms. The resulting ions can be accelerated by electric ...
... Having to carry its own fuel puts a lot of mass burden on an engine in space. This is why NASA is developing other types of engines to minimize fuel mass. An ion thruster uses xenon atoms that have had at least one electron removed from their atoms. The resulting ions can be accelerated by electric ...
No Slide Title
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
The Chemistry of Aqueous Systems
... 77) Be able to identify, name and write the correct formulas for the oxo-acids of the halogens; 78) Be able to list the 7 mechanisms for acid formation, as well as at least one example thereof; 79) Be able to list the 5 mechanisms for base formation, as well as at least one example thereof; 80) Be a ...
... 77) Be able to identify, name and write the correct formulas for the oxo-acids of the halogens; 78) Be able to list the 7 mechanisms for acid formation, as well as at least one example thereof; 79) Be able to list the 5 mechanisms for base formation, as well as at least one example thereof; 80) Be a ...
the chemistry of life: organic and biological chemistry
... Although biological systems are almost unimaginably complex, they are nevertheless constructed of molecules of quite modest size, put together in nature to form a host of complex, interacting structures. The example of phenylalanine and PKU illustrates the point that to understand biology, we need t ...
... Although biological systems are almost unimaginably complex, they are nevertheless constructed of molecules of quite modest size, put together in nature to form a host of complex, interacting structures. The example of phenylalanine and PKU illustrates the point that to understand biology, we need t ...
FREE Sample Here
... Full file at http://textbooktestbank.eu/Campbell-Biology-with-MasteringBiology-9th-Edition-Test-Bank-Reece Campbell's Biology, 9e (Reece et al.) Chapter 2 The Chemical Context of Life This chapter presents basic chemical principles for understanding the chemical context of living organisms, from ato ...
... Full file at http://textbooktestbank.eu/Campbell-Biology-with-MasteringBiology-9th-Edition-Test-Bank-Reece Campbell's Biology, 9e (Reece et al.) Chapter 2 The Chemical Context of Life This chapter presents basic chemical principles for understanding the chemical context of living organisms, from ato ...
Net ionic equation
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
FREE Sample Here
... Full file at http://testbank360.eu/test-bank-campbell-biology-with-masteringbiology-9th-edition-reece Campbell's Biology, 9e (Reece et al.) Chapter 2 The Chemical Context of Life This chapter presents basic chemical principles for understanding the chemical context of living organisms, from atomic s ...
... Full file at http://testbank360.eu/test-bank-campbell-biology-with-masteringbiology-9th-edition-reece Campbell's Biology, 9e (Reece et al.) Chapter 2 The Chemical Context of Life This chapter presents basic chemical principles for understanding the chemical context of living organisms, from atomic s ...
Powerpoint
... Bond enthalpy is usually applied for gaseous molecules because molecules are isolated in gaseous state. In solid and liquid states, molecules are held by each other by intermolecular forces. The importance of bond enthalpy relies on the fact that it can be calculated accurately and there are some ex ...
... Bond enthalpy is usually applied for gaseous molecules because molecules are isolated in gaseous state. In solid and liquid states, molecules are held by each other by intermolecular forces. The importance of bond enthalpy relies on the fact that it can be calculated accurately and there are some ex ...
surface chemistry - einstein classes
... system as a whole is electrically natural. Colloidal particles having similar charge, repel each other, and do not combine to form bigger particles and thus solution is stable and particles do not settle down. When an electric current is passed through a colloidal solution the solid particles and th ...
... system as a whole is electrically natural. Colloidal particles having similar charge, repel each other, and do not combine to form bigger particles and thus solution is stable and particles do not settle down. When an electric current is passed through a colloidal solution the solid particles and th ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... When metals react they lose electrons or are oxidized, when non-metals react they gain electrons or are reduced. Therefore the reactivity of a metal or non-metal is about how easily it is oxidized or reduced or how strong a reducing or oxidizing agent it is. The strength of an oxidising or reducing ...
... When metals react they lose electrons or are oxidized, when non-metals react they gain electrons or are reduced. Therefore the reactivity of a metal or non-metal is about how easily it is oxidized or reduced or how strong a reducing or oxidizing agent it is. The strength of an oxidising or reducing ...
Experiment 9
... great so that the forces of interionic interaction manifest themselves appreciably even at low concentration of an electrolyte. As a result, the ions are not completely free. This is why the state of ions in a solution is described, in addition to their concentration, by their activity, i.e. the con ...
... great so that the forces of interionic interaction manifest themselves appreciably even at low concentration of an electrolyte. As a result, the ions are not completely free. This is why the state of ions in a solution is described, in addition to their concentration, by their activity, i.e. the con ...
Formation Mechanisms of Naphthalene and
... ≥ 9) may form hydrogenated naphthalenes and then produce naphthalene after consecutive dehydrogenation steps. Alternatively, the reactions involving PES with x = 6 and 7 can lead to naphthalene via hydrogen atom additions if hydrogen atoms are available. One of the reactants usually contains an arom ...
... ≥ 9) may form hydrogenated naphthalenes and then produce naphthalene after consecutive dehydrogenation steps. Alternatively, the reactions involving PES with x = 6 and 7 can lead to naphthalene via hydrogen atom additions if hydrogen atoms are available. One of the reactants usually contains an arom ...
Textbook Answer Keys - Mr. Massey`s Chemistry Pages
... 6. A; the relationship between wavelength, frequency and energy is the greater the energy, the shorter (smaller) the wavelength and the higher the frequency; ultraviolet light is high energy/ short wavelength when compared to visible light; infrared light is lower energy/low frequency compare to vis ...
... 6. A; the relationship between wavelength, frequency and energy is the greater the energy, the shorter (smaller) the wavelength and the higher the frequency; ultraviolet light is high energy/ short wavelength when compared to visible light; infrared light is lower energy/low frequency compare to vis ...
Hydrogen bond dynamics of superheated water and methanol by
... thus relevant to the widespread use of supercritical solvents in industrial reactions and separation science. SCW consists of highly excited molecules9 and is believed to be important in the geological formation of hydrocarbons.10–13 New forms of life have been found at deep-sea hydrothermal vents14 ...
... thus relevant to the widespread use of supercritical solvents in industrial reactions and separation science. SCW consists of highly excited molecules9 and is believed to be important in the geological formation of hydrocarbons.10–13 New forms of life have been found at deep-sea hydrothermal vents14 ...
From Organometallic Zinc and Copper Complexes to Highly
... The pre-catalyst solution was injected into a de-gassed solution of squalane in a continuously stirred tank reactor (CSTR), under an atmosphere of N2 at 298 K. A 3:1 H2:CO2 mixture, at 50 bar, was added and the reactor was heated to 523 K. The reaction was monitored using an in-line GC to detect the ...
... The pre-catalyst solution was injected into a de-gassed solution of squalane in a continuously stirred tank reactor (CSTR), under an atmosphere of N2 at 298 K. A 3:1 H2:CO2 mixture, at 50 bar, was added and the reactor was heated to 523 K. The reaction was monitored using an in-line GC to detect the ...
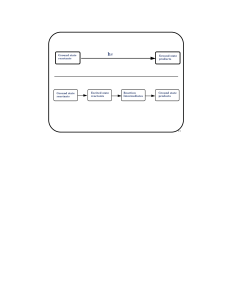
Ground state reactants Ground state products Ground state
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
1984 Advanced Placement Exam
... 26. According to the rate law for the reaction, an increase in the concentration of hydronium ion has what effect on this reaction? (A) The rate of reaction increases. (B) The rate of reaction decreases. (C) The value of the equilibrium constant increases. (D) The value of the equilibrium constant d ...
... 26. According to the rate law for the reaction, an increase in the concentration of hydronium ion has what effect on this reaction? (A) The rate of reaction increases. (B) The rate of reaction decreases. (C) The value of the equilibrium constant increases. (D) The value of the equilibrium constant d ...
Proton Chemical Shift Tensors and Hydrogen Bond Geometry: A 1H
... Proton CS Tensors of the Stationary Water Molecules. It is clear from above discussion that the rapid flipping motion of the water molecule results in aVeraged 1H CS tensors to be measured through experiments. However, it is more desirable to obtain 1H CS tensors for the stationary water molecules, ...
... Proton CS Tensors of the Stationary Water Molecules. It is clear from above discussion that the rapid flipping motion of the water molecule results in aVeraged 1H CS tensors to be measured through experiments. However, it is more desirable to obtain 1H CS tensors for the stationary water molecules, ...
Name: Period:______ Let`s make some sandwiches! Introduction: If
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
Document
... One of the many negative consequences of ocean acidification is thought to be the disruption of the chemistry of ocean water, leading to things such as a decreased rate of calcification amongst many marine organisms. For example, one of the main compounds from which coral build their structure is ca ...
... One of the many negative consequences of ocean acidification is thought to be the disruption of the chemistry of ocean water, leading to things such as a decreased rate of calcification amongst many marine organisms. For example, one of the main compounds from which coral build their structure is ca ...
coordination compounds - Ahlcon Public School , Mayur Vihar Ph
... The two strands in DNA molecule are held together by hydrogen bonds between purine base of one strand and pyrimidine base of the other and vice-versa. Because of different sizes and geometries of the bases, the only possible pairing in DNA are G (guanine) and C (Cytosine), through three H – bonds i. ...
... The two strands in DNA molecule are held together by hydrogen bonds between purine base of one strand and pyrimidine base of the other and vice-versa. Because of different sizes and geometries of the bases, the only possible pairing in DNA are G (guanine) and C (Cytosine), through three H – bonds i. ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... When metals react they lose electrons or are oxidized, when non-metals react they gain electrons or are reduced. Therefore the reactivity of a metal or non-metal is about how easily it is oxidized or reduced or how strong a reducing or oxidizing agent it is. The strength of an oxidising or reducing ...
... When metals react they lose electrons or are oxidized, when non-metals react they gain electrons or are reduced. Therefore the reactivity of a metal or non-metal is about how easily it is oxidized or reduced or how strong a reducing or oxidizing agent it is. The strength of an oxidising or reducing ...