Chapter 11 Chemical Reactions
... For some, we will be able to: c) predict whether or not they will happen at all. How? We recognize them by their reactants ...
... For some, we will be able to: c) predict whether or not they will happen at all. How? We recognize them by their reactants ...
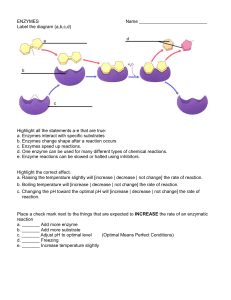
ENZYMES
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
Chapter 2 – Chemical Composition of the Body
... • Bonds formed between the hydrogen end (+ charged) of a polar molecule and the – end of any other polar molecule or highly electronegative atom (e.g. P, N, O) are called hydrogen bonds. • These hydrogen bonds are very important because they alter the physical and chemical properties of many molec ...
... • Bonds formed between the hydrogen end (+ charged) of a polar molecule and the – end of any other polar molecule or highly electronegative atom (e.g. P, N, O) are called hydrogen bonds. • These hydrogen bonds are very important because they alter the physical and chemical properties of many molec ...
IntroRedoxDCIAns
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
Introduction to Oxidation Reduction
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
The five main types of redox reactions are combination
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
Acids and Bases and Aqueous Equilibria
... The value of the hydrogen ion concentration will accordingly be expressed by the hydrogen ion based on the normality factor of the solution used, and this factor will have the form of a negative power of 10. Since in the following section I usually refer to this, I will explain here that I use the n ...
... The value of the hydrogen ion concentration will accordingly be expressed by the hydrogen ion based on the normality factor of the solution used, and this factor will have the form of a negative power of 10. Since in the following section I usually refer to this, I will explain here that I use the n ...
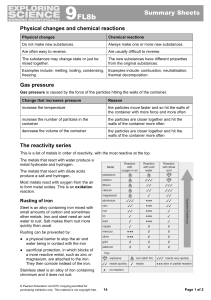
Determination of the reaction order Determination of the reaction
... equation. Catalysts actually react (take part in reactions), chging their mechanisms (othwerwise, how can they influence the reaction rate?). ...
... equation. Catalysts actually react (take part in reactions), chging their mechanisms (othwerwise, how can they influence the reaction rate?). ...
9F Reactivity - Parrs Wood High School
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
Chem Reactions (and Balancing Equations)
... • If the compound has more than two elements you must be given one of the products • The other product will be from the missing pieces • NiCO3 (aq) ...
... • If the compound has more than two elements you must be given one of the products • The other product will be from the missing pieces • NiCO3 (aq) ...
Chap 2.1 Notes - Nature of Matter
... Bonding – is chemical reaction between atoms to create compounds. 2 Types of Bonding 1) Covalent – the sharing of 1 or more pairs of electrons between two atoms. 2) Ionic – attractions between oppositely charged ions – involves the gain or lose of electrons. ...
... Bonding – is chemical reaction between atoms to create compounds. 2 Types of Bonding 1) Covalent – the sharing of 1 or more pairs of electrons between two atoms. 2) Ionic – attractions between oppositely charged ions – involves the gain or lose of electrons. ...
Intro to Chemical Equations note
... When any halogen (Group 17), hydrogen, oxygen, or nitrogen are by themselves in an equation, they are shown as DIATOMIC ELEMENTS. H2 O2 N2 F2 Cl2 Br2 I2 ...
... When any halogen (Group 17), hydrogen, oxygen, or nitrogen are by themselves in an equation, they are shown as DIATOMIC ELEMENTS. H2 O2 N2 F2 Cl2 Br2 I2 ...
Paper
... 7. A chemical equilibrium is established when eleven moles of hydrogen and eleven moles of iodine are mixed at a temperature of 764 K. Initially the colour of the mixture is deep purple due to the high concentration of iodine vapour. The purple colour fades and when equilibrium is established the co ...
... 7. A chemical equilibrium is established when eleven moles of hydrogen and eleven moles of iodine are mixed at a temperature of 764 K. Initially the colour of the mixture is deep purple due to the high concentration of iodine vapour. The purple colour fades and when equilibrium is established the co ...
UN1001: Section 11: Hydrogen Effects
... These hydrides are themselves brittle, and crack, and the crack can propagate through the material, with more hydride progressively precipitating at the crack tip. ...
... These hydrides are themselves brittle, and crack, and the crack can propagate through the material, with more hydride progressively precipitating at the crack tip. ...
PowerPoint Overview for Introduction
... whereas those appearing only at the level of parts per million or less are referred to as micronutrients. These nutrients perform various functions, including the building of bones and cell structures, regulating the body's pH, carrying charge, and driving chemical reactions. ...
... whereas those appearing only at the level of parts per million or less are referred to as micronutrients. These nutrients perform various functions, including the building of bones and cell structures, regulating the body's pH, carrying charge, and driving chemical reactions. ...
Topic 8.4 Acids and Bases The pH Scale
... power of hydrogen’, the scale provides a simple and universal measurement of the amount of hydrogen ions in a solution, which affects its acidity and how it reacts chemically. ...
... power of hydrogen’, the scale provides a simple and universal measurement of the amount of hydrogen ions in a solution, which affects its acidity and how it reacts chemically. ...
CH30S Chemical Reactions Part 2 Unit Review
... 18. How many molecules of methane gas (CH4) would be found in 6.4g of methane at STP? (2.4x1023molecules) 19. A student placed 8.25g of aluminum metal into excess hydrochloric acid (HCl) solution. All of the aluminum reacted to form solid aluminum chloride and hydrogen gas. a. Write the balanced equ ...
... 18. How many molecules of methane gas (CH4) would be found in 6.4g of methane at STP? (2.4x1023molecules) 19. A student placed 8.25g of aluminum metal into excess hydrochloric acid (HCl) solution. All of the aluminum reacted to form solid aluminum chloride and hydrogen gas. a. Write the balanced equ ...
Decomposition Reaction
... very active metals and they react with cold water to produce the hydroxide and hydrogen gas. 3. The next four metals (magnesium - chromium) are considered active metals and they will react with very hot water or steam to form the oxide and hydrogen gas. 4. The oxides of all of these first metals res ...
... very active metals and they react with cold water to produce the hydroxide and hydrogen gas. 3. The next four metals (magnesium - chromium) are considered active metals and they will react with very hot water or steam to form the oxide and hydrogen gas. 4. The oxides of all of these first metals res ...
1 ChE 505 WORKSHOP 1 1. Why are chemical reactions important
... What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
... What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
Chemicals and Their Reactions
... Word vs Chemical Equations? chemical equations provide more detail such as: Chemical formulas of substances involved The ratio of substances involved State of substances involved ...
... Word vs Chemical Equations? chemical equations provide more detail such as: Chemical formulas of substances involved The ratio of substances involved State of substances involved ...
Unit 6 Jeopardy review - Fort Thomas Independent Schools
... DAILY DOUBLE! Chemicals that act as biological catalysts by speeding up reactions in living things. ...
... DAILY DOUBLE! Chemicals that act as biological catalysts by speeding up reactions in living things. ...
Chemical Reactions and Equations
... Be able to recognize synthesis, decomposition, single replacement, double replacement, combustion and neutralization reactions. ...
... Be able to recognize synthesis, decomposition, single replacement, double replacement, combustion and neutralization reactions. ...