Glycolysis
... - thus two ATP used in Phase 1 - substrate level phosphorylationATP production by the direct transfer of phosphate from intermediate ___________________ _____________________________23 ...
... - thus two ATP used in Phase 1 - substrate level phosphorylationATP production by the direct transfer of phosphate from intermediate ___________________ _____________________________23 ...
2.7 DNA Transcription_translation
... and in this theory each polypeptide has its own gene. e.g. haemoglobin is composed of 4 polypeptides (2 of each type) and there is a gene for each type of polypeptide. This theory, like so many in biology has exceptions. e.g. 1) Some genes code for types of RNA which do not produce polypeptides. 2) ...
... and in this theory each polypeptide has its own gene. e.g. haemoglobin is composed of 4 polypeptides (2 of each type) and there is a gene for each type of polypeptide. This theory, like so many in biology has exceptions. e.g. 1) Some genes code for types of RNA which do not produce polypeptides. 2) ...
MIBCB Syllabus
... possess fundamental biochemical knowledge and have the ability to use this knowledge most appropriately as applied to clinical requirements, i.e. diagnosis of disease and planning and monitoring of therapy. Apart from providing a competent laboratory service, the clinical chemist must be able to fun ...
... possess fundamental biochemical knowledge and have the ability to use this knowledge most appropriately as applied to clinical requirements, i.e. diagnosis of disease and planning and monitoring of therapy. Apart from providing a competent laboratory service, the clinical chemist must be able to fun ...
The Body`s Acid / Alkaline Balance
... What does pH mean? - Water (H2O) ionizes into hydrogen (H+) and hydroxyl (OH-) ions, which when in equal proportion have a neutral pH value of 7. ...
... What does pH mean? - Water (H2O) ionizes into hydrogen (H+) and hydroxyl (OH-) ions, which when in equal proportion have a neutral pH value of 7. ...
Completed notes
... Skeletal muscles produce lactic acid when the body cannot supply enough oxygen, such as during periods of strenuous exercise. ...
... Skeletal muscles produce lactic acid when the body cannot supply enough oxygen, such as during periods of strenuous exercise. ...
Fatty acids - Haverford Alchemy
... The Liver, Clearinghouse for Metabolism The liver is the largest reservoir of blood in the body and the largest internal organ, making up about 2.5% of the body’s mass. Blood carrying the end products of digestion enters the liver through the hepatic portal vein before entering general circulation. ...
... The Liver, Clearinghouse for Metabolism The liver is the largest reservoir of blood in the body and the largest internal organ, making up about 2.5% of the body’s mass. Blood carrying the end products of digestion enters the liver through the hepatic portal vein before entering general circulation. ...
1. dia
... Initial velocity should be: P(t) graph must be linear, time has to be short enough. If incubation time ↑, time ↑ for denaturation because of high temperature or pH 2.) pH: optimum depends on the amino acid composition of the enzyme proper ionization is necessary for S – catalytic site interaction; a ...
... Initial velocity should be: P(t) graph must be linear, time has to be short enough. If incubation time ↑, time ↑ for denaturation because of high temperature or pH 2.) pH: optimum depends on the amino acid composition of the enzyme proper ionization is necessary for S – catalytic site interaction; a ...
Chapter 17 Fatty Acid Catabolism
... For each two-carbon increase in the length of a saturated fatty acid chain, how many additional moles of ATP can be formed upon complete oxidation of one mole of the fatty acid to CO2 and H2O? Ans: Each —CH2—CH2— unit yields 14 extra ATP molecules. The two oxidations of the oxidation pathway produc ...
... For each two-carbon increase in the length of a saturated fatty acid chain, how many additional moles of ATP can be formed upon complete oxidation of one mole of the fatty acid to CO2 and H2O? Ans: Each —CH2—CH2— unit yields 14 extra ATP molecules. The two oxidations of the oxidation pathway produc ...
Case 26 The Role of Specific Amino Acids in the Peptide Hormone
... CASE 26 C The Role of Specific Amino Acids in Glucagon in Receptor Binding and Signal Transduction studies have shown that an aspartate residue near the C-terminus of the receptor protein is essential for glucagon binding. Retaining the amino acid residues important for binding while modifying thos ...
... CASE 26 C The Role of Specific Amino Acids in Glucagon in Receptor Binding and Signal Transduction studies have shown that an aspartate residue near the C-terminus of the receptor protein is essential for glucagon binding. Retaining the amino acid residues important for binding while modifying thos ...
File
... o) Electron Configuration: a way of showing where the electrons are found in an atom. Includes the number of electrons found in each quantum level of the atom, arranged in order from lowest to highest energy. p) Orbital: a region in three-dimensional space around the nucleus of an atom where there i ...
... o) Electron Configuration: a way of showing where the electrons are found in an atom. Includes the number of electrons found in each quantum level of the atom, arranged in order from lowest to highest energy. p) Orbital: a region in three-dimensional space around the nucleus of an atom where there i ...
Comparative Visualization of Protein Structure
... unique. A full listing of these side chains is given in figure 6. In a protein, the amino acids (also called residues) are linked together ...
... unique. A full listing of these side chains is given in figure 6. In a protein, the amino acids (also called residues) are linked together ...
1. Most organisms are active in a limited temperature range
... reaction has a very high activation energy reached only at very high temperatures. If the reaction takes place at high temperatures there are two main disadvantages: all the energy is released spontaneously and is lost to the cell as it cannot be trapped high temperatures can damage living molecules ...
... reaction has a very high activation energy reached only at very high temperatures. If the reaction takes place at high temperatures there are two main disadvantages: all the energy is released spontaneously and is lost to the cell as it cannot be trapped high temperatures can damage living molecules ...
BIOLOGICAL OXIDATION
... 1- Chemical theory :It suggests that there is a direct chemical coupling of oxidation and phosphorylation through high-energy intermediate compounds. This theory is not accepted, as postulated high-energy intermediate compounds were never found. 2- Chemiosomotic theory :It suggest that the transfer ...
... 1- Chemical theory :It suggests that there is a direct chemical coupling of oxidation and phosphorylation through high-energy intermediate compounds. This theory is not accepted, as postulated high-energy intermediate compounds were never found. 2- Chemiosomotic theory :It suggest that the transfer ...
Summary
... •The TCA cycle gives complete oxidation of the pyruvate carbons. •And reduction of 4 NAD+ and one FAD. •And phosphorylation of one GDP. •The TCA cycle is a source of biosynthetic precursors. •Pyruvate carboxylase and the glyoxylate cycle replenish precursors •Replenishment of NAD + and FAD are also ...
... •The TCA cycle gives complete oxidation of the pyruvate carbons. •And reduction of 4 NAD+ and one FAD. •And phosphorylation of one GDP. •The TCA cycle is a source of biosynthetic precursors. •Pyruvate carboxylase and the glyoxylate cycle replenish precursors •Replenishment of NAD + and FAD are also ...
Spring 2014 Chemistry Review
... 23) What 2 properties about a molecule do you use to determine the Molecular (VSEPR) shape of a molecule? a. _____________________________ b. _____________________________ 24) Draw the Lewis structure and name the molecular shape for each molecule: BF3 ...
... 23) What 2 properties about a molecule do you use to determine the Molecular (VSEPR) shape of a molecule? a. _____________________________ b. _____________________________ 24) Draw the Lewis structure and name the molecular shape for each molecule: BF3 ...
Khaled Hamarneh Summary
... Ring with 2 oxygen atoms “IN SEQUENTIAL MANNER” the ring’s function is to make an access for electrons ( because they must be donated with hydrocarbon tail ) .. the function of this tail is to pass freely through the membrane ( hydrophobic structure ) .. 1. Can accept 1 or 2 electrons, it may le ...
... Ring with 2 oxygen atoms “IN SEQUENTIAL MANNER” the ring’s function is to make an access for electrons ( because they must be donated with hydrocarbon tail ) .. the function of this tail is to pass freely through the membrane ( hydrophobic structure ) .. 1. Can accept 1 or 2 electrons, it may le ...
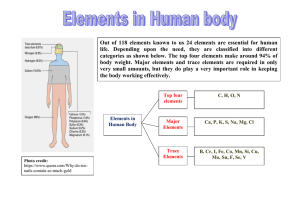
Elements in the Human Body
... Biological role: The heme group present in hemoglobin binds with oxygen and transport it from lungs to tissues. Molecular oxygen is essential for cellular respiration in all organisms. It is used as an electron acceptor in mitochondria present ...
... Biological role: The heme group present in hemoglobin binds with oxygen and transport it from lungs to tissues. Molecular oxygen is essential for cellular respiration in all organisms. It is used as an electron acceptor in mitochondria present ...
Bio 101 General Biology 1
... Syllabus should state what the course grade will be based on, such as tests, quizzes, a comprehensive final exam, and any other assignments made by the ...
... Syllabus should state what the course grade will be based on, such as tests, quizzes, a comprehensive final exam, and any other assignments made by the ...
v semester zoology micro- macro- mega
... It is possible to measure differences between these molecules obtained from different organisms (such as humans, apes, monkeys, prosimians etc.) on a unit scale of amino acids or nucleotides and demonstrate their relationships. As the molecular sequences are heritable, their variations produce molec ...
... It is possible to measure differences between these molecules obtained from different organisms (such as humans, apes, monkeys, prosimians etc.) on a unit scale of amino acids or nucleotides and demonstrate their relationships. As the molecular sequences are heritable, their variations produce molec ...
Poster
... MppP follows typical Michaelis-Menten kinetics where the enzyme and substrate interact at the active site to form the enzymesubstrate complex. After reacting, the substrate has been converted to product and leaves the enzyme intact (Figure 4a). Through the PLP cofactor, MppP converts L-Arg and O2 to ...
... MppP follows typical Michaelis-Menten kinetics where the enzyme and substrate interact at the active site to form the enzymesubstrate complex. After reacting, the substrate has been converted to product and leaves the enzyme intact (Figure 4a). Through the PLP cofactor, MppP converts L-Arg and O2 to ...
HSCE
... The life sciences are changing in ways that have important implications for high school biology. Many of these changes concern our understanding of the largest and the smallest living systems. Molecular biology continues to produce new insights into how living systems work and how they are connected ...
... The life sciences are changing in ways that have important implications for high school biology. Many of these changes concern our understanding of the largest and the smallest living systems. Molecular biology continues to produce new insights into how living systems work and how they are connected ...
Chemistry Review2
... Types of equations or types of reactions: 1. Decomposition: One reactant breaking down into elements or other compounds: 2 PbSO4 2 PbSO3 + 1 O2 2. Combination or synthesis: elements and compounds mixing to form one product 1S8 + 8O2 8SO2 3. Single (re)displacement(SD): The single element(or diato ...
... Types of equations or types of reactions: 1. Decomposition: One reactant breaking down into elements or other compounds: 2 PbSO4 2 PbSO3 + 1 O2 2. Combination or synthesis: elements and compounds mixing to form one product 1S8 + 8O2 8SO2 3. Single (re)displacement(SD): The single element(or diato ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.