Unit 8 Stoichiometry Notes
... • Stoichiometry is a big word for a process that chemist’s use to calculate amounts in reactions. It makes use of the coefficient ratio set up by balanced reaction equations to make connections between the reactants and products in reactions. • Stoichiometry calculates the quantities of reactants an ...
... • Stoichiometry is a big word for a process that chemist’s use to calculate amounts in reactions. It makes use of the coefficient ratio set up by balanced reaction equations to make connections between the reactants and products in reactions. • Stoichiometry calculates the quantities of reactants an ...
Unit 9 Stoichiometry Notes
... Stoichiometry is a big word for a process that chemist’s use to calculate amounts in reactions. It makes use of the coefficient ratio set up by balanced reaction equations to make connections between the reactants and products in reactions. Stoichiometry calculates the quantities of reactants an ...
... Stoichiometry is a big word for a process that chemist’s use to calculate amounts in reactions. It makes use of the coefficient ratio set up by balanced reaction equations to make connections between the reactants and products in reactions. Stoichiometry calculates the quantities of reactants an ...
Unit 10A Stoichiometry Notes
... Stoichiometry is a big word for a process that chemist’s use to calculate amounts in reactions. It makes use of the coefficient ratio set up by balanced reaction equations to make connections between the reactants and products in reactions. Stoichiometry calculates the quantities of reactants an ...
... Stoichiometry is a big word for a process that chemist’s use to calculate amounts in reactions. It makes use of the coefficient ratio set up by balanced reaction equations to make connections between the reactants and products in reactions. Stoichiometry calculates the quantities of reactants an ...
YOU ARE WHAT YOU ABSORB YOU ARE NOT WHAT YOU EAT
... Collagen is the most abundant protein in the human body. It gives the shape and support to all tissues including the skin. ...
... Collagen is the most abundant protein in the human body. It gives the shape and support to all tissues including the skin. ...
Full-Text PDF
... to the cellulose and hemicellulose components. Historically, lignin has been burned as a source of process heat, but this heat is usually in excess of the process energy demands. Current models indicate that development of an economically competitive biorefinery system requires adding value to ligni ...
... to the cellulose and hemicellulose components. Historically, lignin has been burned as a source of process heat, but this heat is usually in excess of the process energy demands. Current models indicate that development of an economically competitive biorefinery system requires adding value to ligni ...
Carnitine-Metabolism and Functions
... histone and a variety of proteins. It is not known whether more than one enzyme is active in protein lysine methylation. Mono-, di-, and trimethylated lysines are all found in different proteins (321), but only the trimethyllysine is converted to carnitine (235). The same derivatives have been isola ...
... histone and a variety of proteins. It is not known whether more than one enzyme is active in protein lysine methylation. Mono-, di-, and trimethylated lysines are all found in different proteins (321), but only the trimethyllysine is converted to carnitine (235). The same derivatives have been isola ...
Biomimetic Reactions Catalyzed by Cyclodextrins and
... enzyme-substrate complex. The substrates examined were rather rigid,84 which helps accelerate the reaction by holding the ester group precisely over the β-CD secondary side hydroxyl group. However, for 4b with imidazole, a poorer leaving group than the p-nitrophenoxide ion in 4a, the acceleration wa ...
... enzyme-substrate complex. The substrates examined were rather rigid,84 which helps accelerate the reaction by holding the ester group precisely over the β-CD secondary side hydroxyl group. However, for 4b with imidazole, a poorer leaving group than the p-nitrophenoxide ion in 4a, the acceleration wa ...
Complete Program - Mathematics and Computer Science
... Chemistry, Wilmington, MA 01887, United StatesWarner Babcock Institute for Green Chemistry, Wilmington, MA 01887, United States Imagine if all consumers, all retailers and all manufacturers insisted on buying and selling only non-toxic materials! The unfortunate reality is that, even if this situati ...
... Chemistry, Wilmington, MA 01887, United StatesWarner Babcock Institute for Green Chemistry, Wilmington, MA 01887, United States Imagine if all consumers, all retailers and all manufacturers insisted on buying and selling only non-toxic materials! The unfortunate reality is that, even if this situati ...
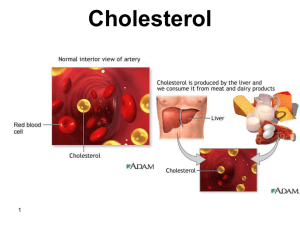
What is Cholesterol?......cont. - Home
... • Cholesterol is oxidized by the liver into a variety of bile acids (about 30 / d). The bile acid synthesis rate average 200-400mg /d. • These in turn are conjugated with glycine, taurine, glucuronic acid, or sulfate. A mixture of conjugated and non-conjugated bile acids along with cholesterol itsel ...
... • Cholesterol is oxidized by the liver into a variety of bile acids (about 30 / d). The bile acid synthesis rate average 200-400mg /d. • These in turn are conjugated with glycine, taurine, glucuronic acid, or sulfate. A mixture of conjugated and non-conjugated bile acids along with cholesterol itsel ...
Regulation of mitochondrial calcium in plants versus animals
... and Marmé, 1980; Akerman and Moore, 1983), others do not (Moore and Bonner, 1977; Martins and Vercesi, 1985). Uptake strictly requires energization and does not take place in the presence of respiratory chain inhibitors such as antimycin A, KCN, and NaN3 (Dieter and Marmé, 1980). Ca2+ import in most ...
... and Marmé, 1980; Akerman and Moore, 1983), others do not (Moore and Bonner, 1977; Martins and Vercesi, 1985). Uptake strictly requires energization and does not take place in the presence of respiratory chain inhibitors such as antimycin A, KCN, and NaN3 (Dieter and Marmé, 1980). Ca2+ import in most ...
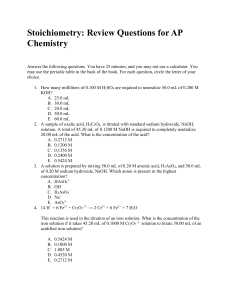
Stoichiometry
... reach from the Sun to Pluto and back 7.5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills. ...
... reach from the Sun to Pluto and back 7.5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills. ...
volume 2 - PianetaChimica
... This publication contains the competition problems (Volume 2) from the 21st – 40th International Chemistry Olympiads (IChO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty IChOs. ...
... This publication contains the competition problems (Volume 2) from the 21st – 40th International Chemistry Olympiads (IChO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty IChOs. ...
Science Bowl Biological Questions
... BIOL-91; Multiple Choice: Into which of the following acids is glucose broken down in the first stage of carbohydrate metabolism? Is it: a) pyruvic acid (pie-rue-vick acid) b) lactic acid c) hydrochloric acid d) citric acid ANSWER: A -- PYRUVIC ACID BIOL-91; Multiple Choice: Hormones are composed fr ...
... BIOL-91; Multiple Choice: Into which of the following acids is glucose broken down in the first stage of carbohydrate metabolism? Is it: a) pyruvic acid (pie-rue-vick acid) b) lactic acid c) hydrochloric acid d) citric acid ANSWER: A -- PYRUVIC ACID BIOL-91; Multiple Choice: Hormones are composed fr ...
volume 2 - HotNews
... This publication contains the competition problems (Volume 2) from the 21st – 40th International Chemistry Olympiads (ICHO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty ICHOs. ...
... This publication contains the competition problems (Volume 2) from the 21st – 40th International Chemistry Olympiads (ICHO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty ICHOs. ...
Quantum dots: Biological and biomedical applications (PDF
... cause irreversible damage to nucleic acids, enzymes, and cellular components such as mitochondria and both plasma and nuclear membranes [3]. As results from above mentioned, the key step in QDs preparation ensuring the achievement of above mentioned required properties is QDs functionalization. Coat ...
... cause irreversible damage to nucleic acids, enzymes, and cellular components such as mitochondria and both plasma and nuclear membranes [3]. As results from above mentioned, the key step in QDs preparation ensuring the achievement of above mentioned required properties is QDs functionalization. Coat ...
Quantitative Chemical Analysis
... One of our most pressing problems is the need for sources of energy to replace oil. The chart at the right shows that world production of oil per capita has probably already peaked. Oil will play a decreasing role as an energy source and should be more valuable as a raw material than as a fuel. Ther ...
... One of our most pressing problems is the need for sources of energy to replace oil. The chart at the right shows that world production of oil per capita has probably already peaked. Oil will play a decreasing role as an energy source and should be more valuable as a raw material than as a fuel. Ther ...
Linus Pauling Institute
... water may be more bioavailable than manganese in food. However, none of the studies measured dietary manganese, so total manganese intake in these cases is unknown. In the U.S., the EPA recommends 0.05 mg/liter as the maximum allowable manganese concentration in drinking water (33). Additionally, mo ...
... water may be more bioavailable than manganese in food. However, none of the studies measured dietary manganese, so total manganese intake in these cases is unknown. In the U.S., the EPA recommends 0.05 mg/liter as the maximum allowable manganese concentration in drinking water (33). Additionally, mo ...
Supplemental Problems
... mine the ages of objects that were once living, such as wood, bones, and fossils. While alive, living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its ...
... mine the ages of objects that were once living, such as wood, bones, and fossils. While alive, living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its ...
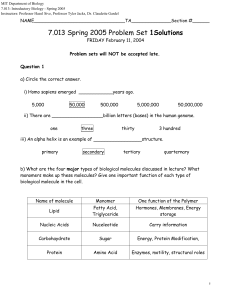
7.013 Spring 2005 Problem Set 1Solutions
... ii) There are __________________billion letters (bases) in the human genome. one ...
... ii) There are __________________billion letters (bases) in the human genome. one ...
Functional Interactions Between the Subunits of the Lactose
... has been determined using freeze-fracture electron microscopy, which suggested that both proteins are present in the bilayer as dimers only (32,123). Additional evidence for this dimeric state of LacS in the membrane came from saturation-transfer electron spin resonance (109). Chemical cross-linking ...
... has been determined using freeze-fracture electron microscopy, which suggested that both proteins are present in the bilayer as dimers only (32,123). Additional evidence for this dimeric state of LacS in the membrane came from saturation-transfer electron spin resonance (109). Chemical cross-linking ...
101-Chem
... General Chemistry-1 Book: Chemistry: The Molecular Nature of Matter, 6E Jespersen/Brady/Hyslop ...
... General Chemistry-1 Book: Chemistry: The Molecular Nature of Matter, 6E Jespersen/Brady/Hyslop ...
Stoichiometry
... 5. Tungsten metal may be prepared by reducing WO3 with H2 gas. How many grams of tungsten may be prepared from 0.0500 mol of WO3 with excess hydrogen? A. 5.58 g B. 0.500 g C. 9.19 g D. 184 g E. 18.4 g 6. Manganese, Mn, forms a number of oxides. A particular oxide is 63.2% Mn. What is the simplest f ...
... 5. Tungsten metal may be prepared by reducing WO3 with H2 gas. How many grams of tungsten may be prepared from 0.0500 mol of WO3 with excess hydrogen? A. 5.58 g B. 0.500 g C. 9.19 g D. 184 g E. 18.4 g 6. Manganese, Mn, forms a number of oxides. A particular oxide is 63.2% Mn. What is the simplest f ...
Polynucleotide Phosphorylase and Mitochondrial
... Tseng, 2007). The pentavalent arsenate (AsV), the prevalent form of inorganic arsenic in the environment, enters the body via the intestinal inorganic phosphate (Pi) transporters (VillaBellosta and Sorribas, 2008). Inside the cells, AsV can impair energy homeostasis by replacing Pi in enzyme reactio ...
... Tseng, 2007). The pentavalent arsenate (AsV), the prevalent form of inorganic arsenic in the environment, enters the body via the intestinal inorganic phosphate (Pi) transporters (VillaBellosta and Sorribas, 2008). Inside the cells, AsV can impair energy homeostasis by replacing Pi in enzyme reactio ...
Manganese Bulletin
... Wound healing is a complex process that requires increased production of collagen. Manganese is required for the activation of prolidase, an enzyme that functions to provide the amino acid, proline, for collagen formation in human skin cells. A genetic disorder known as prolidase deficiency results ...
... Wound healing is a complex process that requires increased production of collagen. Manganese is required for the activation of prolidase, an enzyme that functions to provide the amino acid, proline, for collagen formation in human skin cells. A genetic disorder known as prolidase deficiency results ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.