Chapter 4: Experimental Techniques
... Analysing chemical compounds and confirming their identities is a crucial part of practical chemistry. In addition to applications in research laboratories, analysis is a daily part of scientists’ work in areas such as the pharmaceutical industry, food and drink quality control, environmental monito ...
... Analysing chemical compounds and confirming their identities is a crucial part of practical chemistry. In addition to applications in research laboratories, analysis is a daily part of scientists’ work in areas such as the pharmaceutical industry, food and drink quality control, environmental monito ...
Chapter 5 Powerpoint - Moore Public Schools
... geometric arrangement of atoms, or its crystalline structure. • crystal a solid whose atoms, ions, or molecules are arranged in a regular, repeating pattern • One way that scientists study the structure of crystals is by using X rays. X rays that pass through a crystal and strike a photographic plat ...
... geometric arrangement of atoms, or its crystalline structure. • crystal a solid whose atoms, ions, or molecules are arranged in a regular, repeating pattern • One way that scientists study the structure of crystals is by using X rays. X rays that pass through a crystal and strike a photographic plat ...
KHOA: HÓA HỌC - CCS - Trường Đại học Sư phạm Hà Nội
... throughout a given sample and from one sample to another. A chemical element is a substance comprised of a single type of atom. The elements are the building blocks of our nature. An element is either discovered in nature or synthesized in the laboratory in pure form that cannot be separated into s ...
... throughout a given sample and from one sample to another. A chemical element is a substance comprised of a single type of atom. The elements are the building blocks of our nature. An element is either discovered in nature or synthesized in the laboratory in pure form that cannot be separated into s ...
Modified ketone resin as an epoxy resin curing agent
... The C,H and Br contents of BCHF also agree with the predicted structure. The bromine content of BCHF polymer indicate that the bromination of terminal OH group could not be exist. This is only possible at higher temperature [11]. The reaction of BCHF with hydrazine and its various derivatives was ca ...
... The C,H and Br contents of BCHF also agree with the predicted structure. The bromine content of BCHF polymer indicate that the bromination of terminal OH group could not be exist. This is only possible at higher temperature [11]. The reaction of BCHF with hydrazine and its various derivatives was ca ...
Pharmacy orientation for pre
... • The pharmacist serves as portal of entry into the health care system. • The community pharmacist help assist these patients to find the best health specialty or deal himself with the problem for OTC ( over the counter) drugs. ...
... • The pharmacist serves as portal of entry into the health care system. • The community pharmacist help assist these patients to find the best health specialty or deal himself with the problem for OTC ( over the counter) drugs. ...
Minerals
... slab of a mineral that is different from the rock surrounding it. Often these mineral form where tectonic plates spread apart forming chimneys along the midocean ridge. Other minerals can be seen when the solution evaporates. One example of this is the mineral halite (salt). ...
... slab of a mineral that is different from the rock surrounding it. Often these mineral form where tectonic plates spread apart forming chimneys along the midocean ridge. Other minerals can be seen when the solution evaporates. One example of this is the mineral halite (salt). ...
covalent - Typepad
... c. results when an alkaline-earth metal loses one of its two outermost electrons. d. has more protons than electrons. 4. The elements of the ____ group satisfy the octet rule without forming compounds. a. main c. alkali metal b. noble gas d. alkaline-earth metal 5. Once an atom has a full outermost ...
... c. results when an alkaline-earth metal loses one of its two outermost electrons. d. has more protons than electrons. 4. The elements of the ____ group satisfy the octet rule without forming compounds. a. main c. alkali metal b. noble gas d. alkaline-earth metal 5. Once an atom has a full outermost ...
TextMineralProperties
... manufactured by humans and commonly passed off as minerals: cubic zirconia (an inexpensive diamond substitute), synthetic corundum (the red "ruby", and blue "sapphire" found in inexpensive rings), and industrial diamond (a valuable abrasive). It also excludes a variety of natural substances that are ...
... manufactured by humans and commonly passed off as minerals: cubic zirconia (an inexpensive diamond substitute), synthetic corundum (the red "ruby", and blue "sapphire" found in inexpensive rings), and industrial diamond (a valuable abrasive). It also excludes a variety of natural substances that are ...
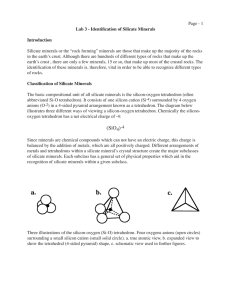
Lab 03 - CEES at TAMIU
... Feldspars are further separated chemically into 2 groups; potassium feldspars and plagioclase feldspars. Potassium feldspars (the most common of which is orthoclase, or more properly, orthoclase feldspar) are mainly pink, but may also be gray, red, orange and rarely green. Close examination of ortho ...
... Feldspars are further separated chemically into 2 groups; potassium feldspars and plagioclase feldspars. Potassium feldspars (the most common of which is orthoclase, or more properly, orthoclase feldspar) are mainly pink, but may also be gray, red, orange and rarely green. Close examination of ortho ...
2(g)
... • RXNS don’t communicate temp. and pressure • RXNS don’t communicate progress or process • RXNS don’t communicate measurable quantities of substances (can’t really weigh or calculate moles, atoms, molecules, ions, etc. without a calculation first) ...
... • RXNS don’t communicate temp. and pressure • RXNS don’t communicate progress or process • RXNS don’t communicate measurable quantities of substances (can’t really weigh or calculate moles, atoms, molecules, ions, etc. without a calculation first) ...
Barbadensis Aloevera
... and lupeol. All these have anti-inflammatory action and lupeol also possesses antiseptic and analgesic properties. ...
... and lupeol. All these have anti-inflammatory action and lupeol also possesses antiseptic and analgesic properties. ...
Brief Review of Liquid Crystals
... phase and the usual melt in cholesterolbenzoate melting. The color of that phase depended on the temperature and vanished in transition to the usual melt but sometimes it remained in the solid phase if the latter was rapidly overcooled. Reinitzer was not only a chemist, he was also proficient in tech ...
... phase and the usual melt in cholesterolbenzoate melting. The color of that phase depended on the temperature and vanished in transition to the usual melt but sometimes it remained in the solid phase if the latter was rapidly overcooled. Reinitzer was not only a chemist, he was also proficient in tech ...
New polyanion-based cathode materials for alkali
... A number of new materials have been discovered through exploratory synthesis with the aim to be studied as the positive electrode (cathode) in Li-ion and Na-ion batteries. The focus has been set on the ease of synthesis, cost and availability of active ingredients in the battery, and decent cycle-li ...
... A number of new materials have been discovered through exploratory synthesis with the aim to be studied as the positive electrode (cathode) in Li-ion and Na-ion batteries. The focus has been set on the ease of synthesis, cost and availability of active ingredients in the battery, and decent cycle-li ...
Mineral Groups
... Feldspar is the most common mineral in the Earth’s crust, so you are very likely to find it in the rocks you collect! It is found it all of the three rock types, but is most common in intrusive igneous rocks like granite where the crystals look white or pink. There are several types of feldspar. Th ...
... Feldspar is the most common mineral in the Earth’s crust, so you are very likely to find it in the rocks you collect! It is found it all of the three rock types, but is most common in intrusive igneous rocks like granite where the crystals look white or pink. There are several types of feldspar. Th ...
First in situ X-ray identification of coesite and retrograde quartz on a
... of the eclogite facies in two areas of Europe: pyrope-quartzite in the Dora Maira Massif of the Western Alps (Chopin 1984) and in dolomite-eclogite from the Western gneiss region of Norway (Smith 1984). In 1990, diamond inclusions were found in garnet from metamorphic rocks derived from crustal mate ...
... of the eclogite facies in two areas of Europe: pyrope-quartzite in the Dora Maira Massif of the Western Alps (Chopin 1984) and in dolomite-eclogite from the Western gneiss region of Norway (Smith 1984). In 1990, diamond inclusions were found in garnet from metamorphic rocks derived from crustal mate ...
SCHOOL OF CHEMICAL SCIENCES
... both the teaching and research laboratories. As such, the graduates can pursue career in public and private companies such as the Malaysian Palm Oil Board (MPOB), the Malaysian Agricultural Research and Development Institute (MARDI), the Forestry Research Institute Malaysia (FRIM) and the Chemistry ...
... both the teaching and research laboratories. As such, the graduates can pursue career in public and private companies such as the Malaysian Palm Oil Board (MPOB), the Malaysian Agricultural Research and Development Institute (MARDI), the Forestry Research Institute Malaysia (FRIM) and the Chemistry ...
Under Choice Based Credit System Proposed syllabus and Scheme of Examination
... Strong, moderate and weak electrolytes, degree of ionization, factors affecting degree of ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for d ...
... Strong, moderate and weak electrolytes, degree of ionization, factors affecting degree of ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for d ...
Benzylamine reacts with nitrous acid to form unstable
... reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement of the hydrogen atom of −NH 2 or > NH group by the acetyl group, which in turn leads to the production of amides. To shift the equilibrium to the right hand side, the ...
... reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement of the hydrogen atom of −NH 2 or > NH group by the acetyl group, which in turn leads to the production of amides. To shift the equilibrium to the right hand side, the ...
IB Chemistry HL Topic5 Questions 1. Which
... Calculate the standard free energy change at 298 K, ΔGӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use ΔS Ө = –360 J K–1 (this is not the correct value). ...
... Calculate the standard free energy change at 298 K, ΔGӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use ΔS Ө = –360 J K–1 (this is not the correct value). ...
File - Roden`s AP Chemistry
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
1970 - Warren County Schools
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
Chapter 4—Rocks and Minerals: Documents that Record Earth`s
... 5. Partial melting occurs where pressure is slightly relieved and minerals with select melting points will liquefy and mobilize at the expense of others left behind. In fractional crystallization, some crystals form in the liquid magma as it cools. These crystals fall out or are removed thus making ...
... 5. Partial melting occurs where pressure is slightly relieved and minerals with select melting points will liquefy and mobilize at the expense of others left behind. In fractional crystallization, some crystals form in the liquid magma as it cools. These crystals fall out or are removed thus making ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
Molecules, Compounds, and Chemical Equations
... each element in a compound. A molecular formula gives the actual number of atoms of each element in a molecule of a compound. For example, the empirical formula for hydrogen peroxide is HO, but its molecular formula is H2O2. The molecular formula is always a whole-number multiple of the empirical fo ...
... each element in a compound. A molecular formula gives the actual number of atoms of each element in a molecule of a compound. For example, the empirical formula for hydrogen peroxide is HO, but its molecular formula is H2O2. The molecular formula is always a whole-number multiple of the empirical fo ...