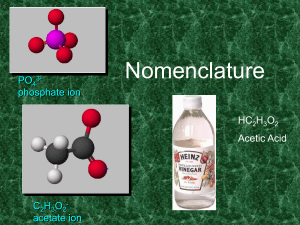
Chapter 3 – part I Sections 1-3
... ions) are these. You will learn later that strong acids and bases are strong electrolytes. • Weak electrolytes are weak conductors, ionic compounds that are insoluble are these. Insoluble = only a few dissolve into ions. You will learn later that weak acids and bases are weak electrolytes. • Non-ele ...
... ions) are these. You will learn later that strong acids and bases are strong electrolytes. • Weak electrolytes are weak conductors, ionic compounds that are insoluble are these. Insoluble = only a few dissolve into ions. You will learn later that weak acids and bases are weak electrolytes. • Non-ele ...
Teacher Materials - Scope, Sequence, and Coordination
... General Background Material. Solutions may be defined as any homogeneous mixture of two or more pure substances. Solutions can be formed from mixtures of substances of one or more phases: gasgas, gas-liquid, gas-solid, liquid-liquid, solid-liquid, and solid-solid are usually listed. Once a solution ...
... General Background Material. Solutions may be defined as any homogeneous mixture of two or more pure substances. Solutions can be formed from mixtures of substances of one or more phases: gasgas, gas-liquid, gas-solid, liquid-liquid, solid-liquid, and solid-solid are usually listed. Once a solution ...
Understanding the Role of Aqueous Solution in Chemical Reactions
... Water is a unique solvent due its structure and reactivity. The ability of a water molecule to form hydrogen bonds is its most important characteristic. In liquid water, each molecule typically donates and accepts two hydrogen bonds. This structure significantly enhances water’s ability to accept or ...
... Water is a unique solvent due its structure and reactivity. The ability of a water molecule to form hydrogen bonds is its most important characteristic. In liquid water, each molecule typically donates and accepts two hydrogen bonds. This structure significantly enhances water’s ability to accept or ...
Chemistry - Bourbon County Schools
... electrons in bonding atoms Explain how ionic and covalent compounds differ Describe the unique features of bonding in carbon compounds Compare the different types of intermolecular forces (e.g., van der Waals, dispersion) Explain and provide examples for dipole moments, bond polarity, and hydrogen ...
... electrons in bonding atoms Explain how ionic and covalent compounds differ Describe the unique features of bonding in carbon compounds Compare the different types of intermolecular forces (e.g., van der Waals, dispersion) Explain and provide examples for dipole moments, bond polarity, and hydrogen ...
iClicker PARTICIPATION Question: Development of the Modern
... In 1803, John Dalton proposed an atomic theory that is still the basis for many of our theories about the atom. 1. All matter is composed of atoms, which are tiny, indivisible particles. 2. A chemical reaction is a rearrangement of atoms to form different compounds. Atoms are neither created nor des ...
... In 1803, John Dalton proposed an atomic theory that is still the basis for many of our theories about the atom. 1. All matter is composed of atoms, which are tiny, indivisible particles. 2. A chemical reaction is a rearrangement of atoms to form different compounds. Atoms are neither created nor des ...
Hydrogen, Alkalis, and Alkaline Earths
... Same overall result as burning methane: same energy out, same CO2 out. To be clean, H2 must come from something other than fossil fuels. ...
... Same overall result as burning methane: same energy out, same CO2 out. To be clean, H2 must come from something other than fossil fuels. ...
Grade 9 Academic Science
... 42. What is cytokinesis? What happens during cytokinesis? 43. Give 2 reasons why it is important for cells to remain small. 44. What is a mutation? Are they always bad? Give an example. 45. What is a tumour? How is a tumour related to a mutation? Are tumours always bad? 46. What is a transgenic or g ...
... 42. What is cytokinesis? What happens during cytokinesis? 43. Give 2 reasons why it is important for cells to remain small. 44. What is a mutation? Are they always bad? Give an example. 45. What is a tumour? How is a tumour related to a mutation? Are tumours always bad? 46. What is a transgenic or g ...
Unit 1 Review, pages 138–145
... 79. (a) Hydrogen is included in the same column of the periodic table as the alkali metals because it contains one valence electron, as alkali metals do. (b) Hydrogen is not considered to be an alkali metal because it does not have the same physical properties as the alkali metals. 80. (a) The peri ...
... 79. (a) Hydrogen is included in the same column of the periodic table as the alkali metals because it contains one valence electron, as alkali metals do. (b) Hydrogen is not considered to be an alkali metal because it does not have the same physical properties as the alkali metals. 80. (a) The peri ...
File
... 2. The oxidation number of a monatomic ion equals the charge on the ion. Example: Mg2+ has the oxidation number of +2. 3. The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compoun ...
... 2. The oxidation number of a monatomic ion equals the charge on the ion. Example: Mg2+ has the oxidation number of +2. 3. The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compoun ...
Chemistry Readings
... When a metal reacts with a non-metal, the metal will lose electrons to form a positive ion while the non-metal will gain electrons forming a negative ion. Together they form an ionic compound. This is the reaction between Magnesium and Oxygen. Magnesium is in Group IIA. A Magnesium atom will lose 2 ...
... When a metal reacts with a non-metal, the metal will lose electrons to form a positive ion while the non-metal will gain electrons forming a negative ion. Together they form an ionic compound. This is the reaction between Magnesium and Oxygen. Magnesium is in Group IIA. A Magnesium atom will lose 2 ...
Grade 11 Unit 8 - Amazon Web Services
... uniqueness of the carbon atom is the basis of all life. The choice of this atom with its designed characteristics is indeed a mark of an omnipotent Creator. No work of chance could have happened upon the combination necessary to produce life. David proclaimed in Psalm 19:1, “The heavens declare the ...
... uniqueness of the carbon atom is the basis of all life. The choice of this atom with its designed characteristics is indeed a mark of an omnipotent Creator. No work of chance could have happened upon the combination necessary to produce life. David proclaimed in Psalm 19:1, “The heavens declare the ...
Date - PetyaPisanScienceAQ
... Physical Differences: Magnesium is not a good conductor of electricity but copper is a great conductor of electricity and that is why they use it in cables. ...
... Physical Differences: Magnesium is not a good conductor of electricity but copper is a great conductor of electricity and that is why they use it in cables. ...
4/page
... number used to produce it. Addition and subtraction: final answer has the same # of digits to the right of the decimal point as the number with the least # of digits to the right. ...
... number used to produce it. Addition and subtraction: final answer has the same # of digits to the right of the decimal point as the number with the least # of digits to the right. ...
Name Date
... molecule - the smallest part of a compound that still has all of the properties of that compound (Contains at least 2 atoms) H2O ...
... molecule - the smallest part of a compound that still has all of the properties of that compound (Contains at least 2 atoms) H2O ...
Chapter 6.2 Notes
... - because they do not form individual molecules, to write the chemical formulas use the smallest ratio of one ion to another, called the formula unit NaCl 1:1 Na2O 2:1 AlBr3 1:3 - smallest ratio means they will not be divisible by each other and get a whole number - will never have an ionic compound ...
... - because they do not form individual molecules, to write the chemical formulas use the smallest ratio of one ion to another, called the formula unit NaCl 1:1 Na2O 2:1 AlBr3 1:3 - smallest ratio means they will not be divisible by each other and get a whole number - will never have an ionic compound ...
CHAPtER 9 Properties and reactions of organic compounds
... essentially infinite because there are so many combinations of organic compounds. However, certain general patterns involving addition, decomposition, combination, substitution or rearrangement of atoms or groups of atoms can be used to describe many common and useful reactions. It is not unusual to ...
... essentially infinite because there are so many combinations of organic compounds. However, certain general patterns involving addition, decomposition, combination, substitution or rearrangement of atoms or groups of atoms can be used to describe many common and useful reactions. It is not unusual to ...
Chapter 1 Matter and Energy Classifying Matter – An Exercise
... The general approach to enter numbers expressed in scientific notation into your calculator is: 1. Enter the coefficient, including the decimal point. 2. Press the EE or EXP (depending on your calculator model) to express the exponent. This button (EE or EXP) basically stands for “x 10—”. 3. Enter t ...
... The general approach to enter numbers expressed in scientific notation into your calculator is: 1. Enter the coefficient, including the decimal point. 2. Press the EE or EXP (depending on your calculator model) to express the exponent. This button (EE or EXP) basically stands for “x 10—”. 3. Enter t ...
Additional Review
... o all of matter is some combination of these four elements Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did di ...
... o all of matter is some combination of these four elements Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did di ...
Chemistry - Gildredge House
... understanding of different areas of Chemistry and how they relate to each other. Students gain essential practical skills as well as a deep knowledge and understanding of scientific methods and competence in a variety of mathematical and problem solving skills. The course is designed and assessed ag ...
... understanding of different areas of Chemistry and how they relate to each other. Students gain essential practical skills as well as a deep knowledge and understanding of scientific methods and competence in a variety of mathematical and problem solving skills. The course is designed and assessed ag ...
Atoms and Elements: Are they Related?
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...