Energy and Matter in Chemical Change Science 10
... • Scientists use an experiment to search for cause and effect relationships in nature. In other words, they design an experiment so that changes to one item cause something else to vary in a ...
... • Scientists use an experiment to search for cause and effect relationships in nature. In other words, they design an experiment so that changes to one item cause something else to vary in a ...
Day 72 TYPES OF CHEMICAL REACTIONS
... a) write a description of the reaction type on the left side b) an example of that type of reaction using elements/compounds and an example of the reaction type using the letters A, B, C and/or D on the right c) And three examples of the reaction ...
... a) write a description of the reaction type on the left side b) an example of that type of reaction using elements/compounds and an example of the reaction type using the letters A, B, C and/or D on the right c) And three examples of the reaction ...
- 1 - Special Sessions Outline Presentation Style S1: Water
... Toshiro Nagase: [email protected] properties, and also the adsorption and reaction of mineral surfaces. Masaya Suzuki: [email protected] We would like to encourage authors to submit papers on the application of minerals. This session aims to present the latest advances in the min ...
... Toshiro Nagase: [email protected] properties, and also the adsorption and reaction of mineral surfaces. Masaya Suzuki: [email protected] We would like to encourage authors to submit papers on the application of minerals. This session aims to present the latest advances in the min ...
Growth Of Zinc Oxide Crystals By Accelerated Evoporation
... (2nd B), now 12th, group). The native doping of ...
... (2nd B), now 12th, group). The native doping of ...
Wizard Test Maker
... North Carolina Standard Course of Study for Physical Science. Proceed with the test on the next page. ...
... North Carolina Standard Course of Study for Physical Science. Proceed with the test on the next page. ...
AP Chemistry Syllabus - Tuloso
... Knowledge of specific facts of chemistry is essential for an understanding of principles and concepts. These descriptive facts, including the chemistry involved in environmental and societal issues, should not be isolated from the principles being studied but should be taught throughout the course t ...
... Knowledge of specific facts of chemistry is essential for an understanding of principles and concepts. These descriptive facts, including the chemistry involved in environmental and societal issues, should not be isolated from the principles being studied but should be taught throughout the course t ...
CHEMISTRY OF MAIN GROUP ELEMENTS Classification -1 s
... HYDRIDES: Except Pb all other elements form hydrides of the formula MH4. Their stability decreases down the group. Carbon forms Hydrocarbons, Silicon forms Silanes, and Germanium forms Germanes. Preparation of ultra pure Silicon: SiH4 Pyrolysis → Si + 2H2. The silicon thus obtained is further purifi ...
... HYDRIDES: Except Pb all other elements form hydrides of the formula MH4. Their stability decreases down the group. Carbon forms Hydrocarbons, Silicon forms Silanes, and Germanium forms Germanes. Preparation of ultra pure Silicon: SiH4 Pyrolysis → Si + 2H2. The silicon thus obtained is further purifi ...
Organic Chemistry and Medicine
... Organic chemistry is typically taught from a chemical point of view Organic chemistry is the one course EVERYONE has heard of and many DREAD taking Organic chemistry is usually a “make it or break it” class for pre-med students ...
... Organic chemistry is typically taught from a chemical point of view Organic chemistry is the one course EVERYONE has heard of and many DREAD taking Organic chemistry is usually a “make it or break it” class for pre-med students ...
Unit 1 Cycle 2: Interactions and Energy
... Since the chemical formulas, molar masses, and characteristic physical properties are clearly different for a starting material (calcium carbonate) and a product (carbon dioxide), this was a chemical change. ...
... Since the chemical formulas, molar masses, and characteristic physical properties are clearly different for a starting material (calcium carbonate) and a product (carbon dioxide), this was a chemical change. ...
elements in a family have the same number of
... Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. All the noble gases are found in small amounts in the earth's ...
... Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. All the noble gases are found in small amounts in the earth's ...
MERIDIAN PUBLIC SCHOOL DISTRICT
... 2. Demonstrate an understanding of the atomic model of matter by explaining atomic structure and chemical bonding. b. Research and explain crucial contributions and critical experiments of Dalton, Thomson, Rutherford, Bohr, de Broglie, and Schrődinger and describe how each discovery contributed to t ...
... 2. Demonstrate an understanding of the atomic model of matter by explaining atomic structure and chemical bonding. b. Research and explain crucial contributions and critical experiments of Dalton, Thomson, Rutherford, Bohr, de Broglie, and Schrődinger and describe how each discovery contributed to t ...
The microstructure of geopolymers synthesized from industrial wastes
... cement, the CO2 released from the world’s cement kilns could also be significantly reduced. The benefits are thus social, environmental and, not least, financial. The microstructure of such systems has been even less well examined, hindered in part by differences in nomenclature. Some researchers fa ...
... cement, the CO2 released from the world’s cement kilns could also be significantly reduced. The benefits are thus social, environmental and, not least, financial. The microstructure of such systems has been even less well examined, hindered in part by differences in nomenclature. Some researchers fa ...
Synergic Role of Self-Interstitials and Vacancies in Indium Melting
... The investigated material was In with purity of 99.999 wt %. All the samples for HT-XRD measurements have been prepared by slowly cooling from the melt for minimizing the defective structures. Two sets of samples (A and B) with different grain orientation have been examined: set A had a strong {002} ...
... The investigated material was In with purity of 99.999 wt %. All the samples for HT-XRD measurements have been prepared by slowly cooling from the melt for minimizing the defective structures. Two sets of samples (A and B) with different grain orientation have been examined: set A had a strong {002} ...
V-shaped oxydiphthalimides: side-chain engineering - IISER
... mechanism of CIEE is ascribed to the twisted conformation of the molecules in the crystalline state that prevents strong intermolecular π–π interaction. Restricted intramolecular rotations in the crystalline state can also contribute to CIEE. As a consequence, the photoexcited luminogens decay to th ...
... mechanism of CIEE is ascribed to the twisted conformation of the molecules in the crystalline state that prevents strong intermolecular π–π interaction. Restricted intramolecular rotations in the crystalline state can also contribute to CIEE. As a consequence, the photoexcited luminogens decay to th ...
Cornell Notes Topic/Objective: Name: Minerals and their
... A compound is a substance made up of __________________________elements that have been ______________________. An example of a compound is ________________. NATIVE ELEMENTS A mineral made up of only ________________element is called a native element. CRYSTALS A crystal is a _____________whose atoms, ...
... A compound is a substance made up of __________________________elements that have been ______________________. An example of a compound is ________________. NATIVE ELEMENTS A mineral made up of only ________________element is called a native element. CRYSTALS A crystal is a _____________whose atoms, ...
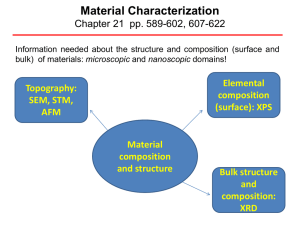
Material Characterization
... number of electrons that escape from the top 1 to 10 nm of the material being analyzed. XPS requires ultra high vacuum (UHV) conditions. ...
... number of electrons that escape from the top 1 to 10 nm of the material being analyzed. XPS requires ultra high vacuum (UHV) conditions. ...
Chapter 3: Atoms: The Building Blocks of Matter
... intermediates, collision theory, activation energy, activated complex, catalyst,, ratedetermining step Essential Questions and Content: Distinguish between heat and temperature. Define the units of heat energy. Perform specific heat calculations. Define the change in enthalpy as the heat of ...
... intermediates, collision theory, activation energy, activated complex, catalyst,, ratedetermining step Essential Questions and Content: Distinguish between heat and temperature. Define the units of heat energy. Perform specific heat calculations. Define the change in enthalpy as the heat of ...
Physical Science - Cabot Public Schools
... 2a. Essential Question - How does balancing chemical equations show the conservation of mass? C.3.PS.2 C.3.PS.3 ...
... 2a. Essential Question - How does balancing chemical equations show the conservation of mass? C.3.PS.2 C.3.PS.3 ...
Florida`s - Wavefunction, Inc.
... A. Energy is involved in all physical and chemical processes. It is conserved, and can be transformed from one form to another and into work. At the atomic and nuclear levels energy is not continuous but exists in discrete amounts. Energy and mass are related through Einstein's equation E=mc2. B. T ...
... A. Energy is involved in all physical and chemical processes. It is conserved, and can be transformed from one form to another and into work. At the atomic and nuclear levels energy is not continuous but exists in discrete amounts. Energy and mass are related through Einstein's equation E=mc2. B. T ...
summerpp_4
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
Chapter 4
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
Ionic Bonding - petersonORHS
... representing the name given) CaClO3 Step 2 Label charges Ca+2 ClO3-1 Step 3 Write subscripts that balance the charges Ca(ClO3)2 Step 4: Erase charges from your formula ...
... representing the name given) CaClO3 Step 2 Label charges Ca+2 ClO3-1 Step 3 Write subscripts that balance the charges Ca(ClO3)2 Step 4: Erase charges from your formula ...
Physical Properties of the Gemstones
... The specific gravity of a gemstone is the ratio of the weight of the material to the weight of the same volume of water at a temperature of 4 degrees Celsius. In general, minerals composed of heavy elements will have a higher specific gravity than those composed of lighter elements, although bonding ...
... The specific gravity of a gemstone is the ratio of the weight of the material to the weight of the same volume of water at a temperature of 4 degrees Celsius. In general, minerals composed of heavy elements will have a higher specific gravity than those composed of lighter elements, although bonding ...
Review for Physical Science Test #2
... 1. Compounds are made of ______________________ of elements that are _______________________________ together. 2. What are two ways that atoms can be bonded together? (Hint: both have to do with electrons.) ...
... 1. Compounds are made of ______________________ of elements that are _______________________________ together. 2. What are two ways that atoms can be bonded together? (Hint: both have to do with electrons.) ...