Document
... form [Tc2Cl8]3– makes it formally Tc(2.5). The Tc-Tc bond length may be controlled by two important factors: bond order and Coulombic repulsion between the formally positively charged Tc ions. The bond order is clearly greater in the 2- ion, but not by much; it also involves a relatively weak delta- ...
... form [Tc2Cl8]3– makes it formally Tc(2.5). The Tc-Tc bond length may be controlled by two important factors: bond order and Coulombic repulsion between the formally positively charged Tc ions. The bond order is clearly greater in the 2- ion, but not by much; it also involves a relatively weak delta- ...
Document
... 12 One pink solid has the formula CoCl3 5NH3 H2O. A solution of this salt is also pink and rapidly gives 3 mol AgCI on titration with silver nitrate solution. When the pink solid is heated, it loses 1 mol H20 to give a purple solid with the same ratio at NH3:Cl:Co. The purple solid releases two of i ...
... 12 One pink solid has the formula CoCl3 5NH3 H2O. A solution of this salt is also pink and rapidly gives 3 mol AgCI on titration with silver nitrate solution. When the pink solid is heated, it loses 1 mol H20 to give a purple solid with the same ratio at NH3:Cl:Co. The purple solid releases two of i ...
5.3.1 Transition Elements 2012
... The first row of transition metals runs from scandium to zinc. All of the elements in this row have outermost electrons in their 3d sub-shells. The 4s sub-shell is at a lower energy than the 3d sub-shells and so the 4s sub shell fills before the 3d sub-shell. A d block element is one which has its h ...
... The first row of transition metals runs from scandium to zinc. All of the elements in this row have outermost electrons in their 3d sub-shells. The 4s sub-shell is at a lower energy than the 3d sub-shells and so the 4s sub shell fills before the 3d sub-shell. A d block element is one which has its h ...
Periodic Table
... Transition metal complexes with unpaired electrons exhibit simple paramagnetism. The degree of paramagnetism depends on the number of unpaired electrons ...
... Transition metal complexes with unpaired electrons exhibit simple paramagnetism. The degree of paramagnetism depends on the number of unpaired electrons ...
TRANSITION ELEMENTS
... Formation of Coloured Compounds Most of the compounds and complexes of transition elements are coloured. The colour of these compounds can often be related to incompletely filled d-orbitals in the transition metal ion. The outer electronic orbitals of transition metal ions have only small energy dif ...
... Formation of Coloured Compounds Most of the compounds and complexes of transition elements are coloured. The colour of these compounds can often be related to incompletely filled d-orbitals in the transition metal ion. The outer electronic orbitals of transition metal ions have only small energy dif ...
壹 - 國立彰化師範大學圖書館
... 1. Write octet structure (including formal charges, bond order, and molecular shape) for Al2Cl6, SnCl3-, BrF4-. (6 %) 2. The hypofluorite ion, OF-, is quite unstable compared to OCl-. (a) Draw a molecular orbital energy level diagram for OF-. (b) What is the bond order in OF-, and how many unpaired ...
... 1. Write octet structure (including formal charges, bond order, and molecular shape) for Al2Cl6, SnCl3-, BrF4-. (6 %) 2. The hypofluorite ion, OF-, is quite unstable compared to OCl-. (a) Draw a molecular orbital energy level diagram for OF-. (b) What is the bond order in OF-, and how many unpaired ...
Ligands - chemmybear.com
... The transition metal complex ion, [Co(NH2CH2CH2NH2)2Cl2]+, has an octahedral shape and exists as two geometrical isomers. (a) Draw the structural formulas for the two geometrical isomers. (b) One of the geometrical isomers exists as two optical isomers. Draw the structural formulas for the two optic ...
... The transition metal complex ion, [Co(NH2CH2CH2NH2)2Cl2]+, has an octahedral shape and exists as two geometrical isomers. (a) Draw the structural formulas for the two geometrical isomers. (b) One of the geometrical isomers exists as two optical isomers. Draw the structural formulas for the two optic ...
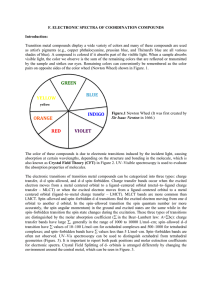
F. ELECTRONIC SPECTRA OF COORDINATION COMPOUNDS
... electron moves from a metal centered orbital to a ligand–centered orbital (metal–to–ligand charge transfer – MLCT) or when the excited electron moves from a ligand–centered orbital to a metal centered orbital (ligand–to–metal charge transfer – LMCT). MLCT bands are more common than LMCT. Spin–allowe ...
... electron moves from a metal centered orbital to a ligand–centered orbital (metal–to–ligand charge transfer – MLCT) or when the excited electron moves from a ligand–centered orbital to a metal centered orbital (ligand–to–metal charge transfer – LMCT). MLCT bands are more common than LMCT. Spin–allowe ...
Slide 1
... The M/M bond order is a function of transition metal electron configuration. The Table below is valid for the case when metal dx2-y2 orbital is used for s-bonding with ligands. ...
... The M/M bond order is a function of transition metal electron configuration. The Table below is valid for the case when metal dx2-y2 orbital is used for s-bonding with ligands. ...
Fe 4 S 4 Cys 4
... • classified by a bands: • a: 580-590 nm • b: 550-560 nm • c: 548-552 nm • (there’s also d and f) • all involved in electron transfer, all CN6 • P450: 450 nm: • Oxygen activation; CN5 ...
... • classified by a bands: • a: 580-590 nm • b: 550-560 nm • c: 548-552 nm • (there’s also d and f) • all involved in electron transfer, all CN6 • P450: 450 nm: • Oxygen activation; CN5 ...
Self-Study_Worksheet II_Basis for Color_ANSWERS
... energies of some of the different d orbitals are affected differently than others, causing them to have a different energy. In the case of an octahedral “field” of ligands (i.e., a geometry of octahedral), it turns out that two of the d orbitals end up having a higher energy than the other three. Th ...
... energies of some of the different d orbitals are affected differently than others, causing them to have a different energy. In the case of an octahedral “field” of ligands (i.e., a geometry of octahedral), it turns out that two of the d orbitals end up having a higher energy than the other three. Th ...
σ−Bonded ligands: Transition Metal Alkyls and Hydrides
... trigonal prismatic where X is not a π donor, not the octahedral structure usually found for ML6 ...
... trigonal prismatic where X is not a π donor, not the octahedral structure usually found for ML6 ...
Brief Guide to the Nomenclature of Inorganic Chemistry
... which the κ-term refers. Multiple identical links to a central atom can be indicated by addition of the appropriate numeral as a superscript between the κ and element symbols (see Table 6). These possibilities are discussed in more detail in the Red Book.13 If the ligating atoms of a ligand are cont ...
... which the κ-term refers. Multiple identical links to a central atom can be indicated by addition of the appropriate numeral as a superscript between the κ and element symbols (see Table 6). These possibilities are discussed in more detail in the Red Book.13 If the ligating atoms of a ligand are cont ...
Brief Guide to the Nomenclature of Inorganic Chemistry
... which the κ-term refers. Multiple identical links to a central atom can be indicated by addition of the appropriate numeral as a superscript between the κ and element symbols (see Table 6). These possibilities are discussed in more detail in the Red Book.13 If the ligating atoms of a ligand are cont ...
... which the κ-term refers. Multiple identical links to a central atom can be indicated by addition of the appropriate numeral as a superscript between the κ and element symbols (see Table 6). These possibilities are discussed in more detail in the Red Book.13 If the ligating atoms of a ligand are cont ...
Mo enzymes Mo enzyme models
... non-superimposable mirror images). In chiral molecules, the methyl groups are often inequivalent in addition to the protons of the CH2 groups that are always diastereotopic. Two groups (in this case protons) are diastereotopic if they have identical atom connectivities but cannot be exchanged by any ...
... non-superimposable mirror images). In chiral molecules, the methyl groups are often inequivalent in addition to the protons of the CH2 groups that are always diastereotopic. Two groups (in this case protons) are diastereotopic if they have identical atom connectivities but cannot be exchanged by any ...
Applications of CFT for Oh Complexes 1. High- and low
... than do dxz, dyz and dxy so they are relatively stabilized • the tetrahedral crystal field (Δt) splitting is less than octahedral splitting (Δoct) because no orbitals point directly at the ligands: Δt = 4/9 Δoct ...
... than do dxz, dyz and dxy so they are relatively stabilized • the tetrahedral crystal field (Δt) splitting is less than octahedral splitting (Δoct) because no orbitals point directly at the ligands: Δt = 4/9 Δoct ...
Molecules 2002
... Thus, 4 was easily prepared from the condensation of four equivalents of 1-(hydroxymethyl)-3,5dimethylpyrazole with one equivalent of p-phenylenediamine under gentle conditions (room temperature, atmospheric pressure, 4-7 days), using anhydrous acetonitrile as solvent. The yield was good (67%) and t ...
... Thus, 4 was easily prepared from the condensation of four equivalents of 1-(hydroxymethyl)-3,5dimethylpyrazole with one equivalent of p-phenylenediamine under gentle conditions (room temperature, atmospheric pressure, 4-7 days), using anhydrous acetonitrile as solvent. The yield was good (67%) and t ...
5.04 Principles of Inorganic Chemistry II
... stabilized by eπ. The same case occurs for a ligand possessing a δ orbital, with the only difference being an energy of stabilization of eδ for the Lδ orbital and the energy of de-stabilization of eδ for the δ metal-based orbitals. SML(δ) is small compared to SML(π) or SML(σ). Moreover, there are fe ...
... stabilized by eπ. The same case occurs for a ligand possessing a δ orbital, with the only difference being an energy of stabilization of eδ for the Lδ orbital and the energy of de-stabilization of eδ for the δ metal-based orbitals. SML(δ) is small compared to SML(π) or SML(σ). Moreover, there are fe ...
CEM 151 – General and Descriptive Chemistry
... Group 1 & Group 2 Chemistry Group 13 chemistry; Bronsted-Lowry and Lewis acids & bases Environmental Chemistry; Group 17 Group 17 & Group 18 Chemistry Group 14 Chemistry Oxygen; Group 16 Chemistry Group 15 chemistry: Nitrogen Introduction to d-block metals; periodic trends Introduction to coordinati ...
... Group 1 & Group 2 Chemistry Group 13 chemistry; Bronsted-Lowry and Lewis acids & bases Environmental Chemistry; Group 17 Group 17 & Group 18 Chemistry Group 14 Chemistry Oxygen; Group 16 Chemistry Group 15 chemistry: Nitrogen Introduction to d-block metals; periodic trends Introduction to coordinati ...
Lab 8: Ligand Substitution in Transition Metal Complexes
... Both [Ni(H2O)6] and [Ni(NH3)6] are complex ions with an octahedral arrangement of donor ligands involved in coordinate covalent bonding around Ni2+. Crystal Field theory suggests that donor electrons from a ligand impose a field on the inner d orbitals of Ni2+, such that the energy required for an e ...
... Both [Ni(H2O)6] and [Ni(NH3)6] are complex ions with an octahedral arrangement of donor ligands involved in coordinate covalent bonding around Ni2+. Crystal Field theory suggests that donor electrons from a ligand impose a field on the inner d orbitals of Ni2+, such that the energy required for an e ...
All contain a transition metal!! All are coordination compounds Many
... Legumes (nitrogen fixers) Radiopharmaceuticals (some) MRI contrast agents ...
... Legumes (nitrogen fixers) Radiopharmaceuticals (some) MRI contrast agents ...
Lecture Notes 9 - La Salle University
... When is one preferred over the other ????? It depends. (P 14,900 cm-1 / e- pair) ...
... When is one preferred over the other ????? It depends. (P 14,900 cm-1 / e- pair) ...
Problems - UCI Chemistry
... e. Now use molecular modeling software to calculate and display the molecular orbitals of [TiH6]2 - . Compare the results with your work in part d and with Figure 10.5, and comment on the similarities and differences. 10.39 Calculate and view the molecular orbitals of the octahedral ion [TiF6]3 - . ...
... e. Now use molecular modeling software to calculate and display the molecular orbitals of [TiH6]2 - . Compare the results with your work in part d and with Figure 10.5, and comment on the similarities and differences. 10.39 Calculate and view the molecular orbitals of the octahedral ion [TiF6]3 - . ...
Fehling`s Test / Benedict`s Test
... Zn2+(aq) ion does not have incompletely filled d-orbital available for d-d electronic transition and therefore it is colourless. Electronic configuration of Cu2+ ion is 3d ...
... Zn2+(aq) ion does not have incompletely filled d-orbital available for d-d electronic transition and therefore it is colourless. Electronic configuration of Cu2+ ion is 3d ...
Ligand
4-3D-balls.png?width=300)
In coordination chemistry, a ligand (/lɪɡənd/) is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from covalent to ionic. Furthermore, the metal-ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic ""ligand.""Metals and metalloids are bound to ligands in virtually all circumstances, although gaseous ""naked"" metal ions can be generated in high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection is a critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.Ligands are classified in many ways like : their charge, their size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity or hapticity). The size of a ligand is indicated by its cone angle.