Organic Chemistry I: Reactions and Overview
... 5.1 Naming Enantiomers via the -R and -S System 1. Each of the four groups attached to the chirality center is assigned a priority of 1, 2, 3, or 4. Priority is assigned on the basis of the atomic number of the atom that is directly attached to the chirality center. The group with the highest atomic ...
... 5.1 Naming Enantiomers via the -R and -S System 1. Each of the four groups attached to the chirality center is assigned a priority of 1, 2, 3, or 4. Priority is assigned on the basis of the atomic number of the atom that is directly attached to the chirality center. The group with the highest atomic ...
Substitution Reactions of Alcohols
... is to convert the alcohol into a sulfonate ester. To make a sulfonate ester, you react an alcohol with an appropriate sulfonyl chloride in the presence of an amine ...
... is to convert the alcohol into a sulfonate ester. To make a sulfonate ester, you react an alcohol with an appropriate sulfonyl chloride in the presence of an amine ...
Converting Alcohols to Alkyl Halides – The Mitsunobu Reaction
... is to convert the alcohol into a sulfonate ester. To make a sulfonate ester, you react an alcohol with an appropriate sulfonyl chloride in the presence of an amine ...
... is to convert the alcohol into a sulfonate ester. To make a sulfonate ester, you react an alcohol with an appropriate sulfonyl chloride in the presence of an amine ...
Chapter 17: Aldehydes and Ketones: Nucleophilic Addition to the
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
Alcohols, Ethers and Epoxides Alcohols contain a hydroxy group (OH)
... Carbocation Rearrangements • Often, when carbocations are intermediates, a less stable carbocation will be converted into a more stable carbocation by a shift of a hydrogen or an alkyl group. This is called a ...
... Carbocation Rearrangements • Often, when carbocations are intermediates, a less stable carbocation will be converted into a more stable carbocation by a shift of a hydrogen or an alkyl group. This is called a ...
E2 reactions
... Polarity is not so important because negative charge is spread over the transition state. ...
... Polarity is not so important because negative charge is spread over the transition state. ...
Chapter 5
... Dehydration: Elimination of a molecule of water from adjacent carbon atoms gives an alkene. ◦ Dehydration is most often brought about by heating an alcohol with either 85% H3PO4 or concentrated H2SO4. ◦ 1° alcohols are the most difficult to dehydrate and require temperatures as high as 180°C. ◦ 2° a ...
... Dehydration: Elimination of a molecule of water from adjacent carbon atoms gives an alkene. ◦ Dehydration is most often brought about by heating an alcohol with either 85% H3PO4 or concentrated H2SO4. ◦ 1° alcohols are the most difficult to dehydrate and require temperatures as high as 180°C. ◦ 2° a ...
5.2. Related mechanisms of halogen chemistry A large variety of
... A large variety of organic reactions involving different halogen species is known within the basic organic chemistry literature (e.g. March, 1992). Those reactions can be divided into two groups: carbon skeleton affecting and functionality modifying reactions. The halogen species discussed here are ...
... A large variety of organic reactions involving different halogen species is known within the basic organic chemistry literature (e.g. March, 1992). Those reactions can be divided into two groups: carbon skeleton affecting and functionality modifying reactions. The halogen species discussed here are ...
Survey on Conditions Catalysis of Chemical Reactions
... carbonyl product. The oxidation state of carbon increases by 2, while the chromium decreases by 3 (it is reduced). Since chromate reagents are a dark orange-red color (VI oxidation state) and chromium III compounds are normally green, the progress of these oxidations is easily observed. Indeed, this ...
... carbonyl product. The oxidation state of carbon increases by 2, while the chromium decreases by 3 (it is reduced). Since chromate reagents are a dark orange-red color (VI oxidation state) and chromium III compounds are normally green, the progress of these oxidations is easily observed. Indeed, this ...
n - TU Chemnitz
... a) S. Kirchmeyer, A. Mertens, G. A. Olah, Synthesis 1983, 500–502; b) A. Hassner, R. Fibiger, A. S. Amarasekara, J. Org. Chem. 1988, 53, 22–27. a) P. Herczegh, M. Zsély, I. Kovács, G. Batta, F. J. Sztaricskai, Tetrahedron Lett. 1990, 31, 1195–1198. b) C. Gauthier, Y. Ramondenc, G. Plé, ...
... a) S. Kirchmeyer, A. Mertens, G. A. Olah, Synthesis 1983, 500–502; b) A. Hassner, R. Fibiger, A. S. Amarasekara, J. Org. Chem. 1988, 53, 22–27. a) P. Herczegh, M. Zsély, I. Kovács, G. Batta, F. J. Sztaricskai, Tetrahedron Lett. 1990, 31, 1195–1198. b) C. Gauthier, Y. Ramondenc, G. Plé, ...
Lab 9 - Academic Computer Center
... The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the three hydride reagents and is easy to use in the lab, because it is soluble in water, methanol and ethanol and does not react with these solvents. Therefore ...
... The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the three hydride reagents and is easy to use in the lab, because it is soluble in water, methanol and ethanol and does not react with these solvents. Therefore ...
fulltext $(function(){PrimeFaces.cw("Tooltip","widget_formSmash_items_resultList_20_j_idt799_0_j_idt801",{id:"formSmash:items:resultList:20:j_idt799:0:j_idt801",widgetVar:"widget_formSmash_items_resultList_20_j_idt799_0_j_idt801",showEffect:"fade",hideEffect:"fade",target:"formSmash:items:resultList:20:j_idt799:0:fullText"});});
... amines with a wide range of primary alcohols, and in the Nheterocyclization of amino alcohols. This reaction resulted in high isolated product yields, even at room temperature and under solvent-free conditions. Amines are important compounds used as agrochemicals, additives and dyes in both the bulk ...
... amines with a wide range of primary alcohols, and in the Nheterocyclization of amino alcohols. This reaction resulted in high isolated product yields, even at room temperature and under solvent-free conditions. Amines are important compounds used as agrochemicals, additives and dyes in both the bulk ...
Secondary alcohols
... Haloalkanes can be made from alcohols through inorganic esters. As an alternative to the acid-catalyzed conversions of alcohols into haloalkanes, a number of inorganic reagents can convert the alcoholic hydroxyl group into a good leaving group under milder conditions. Reaction of PBr3 with a second ...
... Haloalkanes can be made from alcohols through inorganic esters. As an alternative to the acid-catalyzed conversions of alcohols into haloalkanes, a number of inorganic reagents can convert the alcoholic hydroxyl group into a good leaving group under milder conditions. Reaction of PBr3 with a second ...
Common aldehydes and ketones
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
ch12 by dina
... Esters react with two molar equivalents of a Grignard reagent to yield a tertiary alcohol A ketone is formed by the first molar equivalent of Grignard reagent and this immediately reacts with a second equivalent to produce the tertiary alcohol ...
... Esters react with two molar equivalents of a Grignard reagent to yield a tertiary alcohol A ketone is formed by the first molar equivalent of Grignard reagent and this immediately reacts with a second equivalent to produce the tertiary alcohol ...
6-organic - fixurscore
... tertiary carbocation is made stabilised by the electron releasing methyl groups around it. (see alkenes topic for another example of this). Also the bulky methyl groups prevent the hydroxide ion from attacking the halogenoalkane in the same way as the mechanism above ...
... tertiary carbocation is made stabilised by the electron releasing methyl groups around it. (see alkenes topic for another example of this). Also the bulky methyl groups prevent the hydroxide ion from attacking the halogenoalkane in the same way as the mechanism above ...
CHM 222 - Jefferson State Community College
... nomenclature, structure, physical and chemical properties, synthesis, and typical reactions for aliphatic, alicyclic, aromatic, and biological compounds, polymers and their derivatives, with special emphasis on reaction mechanisms, spectroscopy, and stereochemistry. Laboratory is required and will i ...
... nomenclature, structure, physical and chemical properties, synthesis, and typical reactions for aliphatic, alicyclic, aromatic, and biological compounds, polymers and their derivatives, with special emphasis on reaction mechanisms, spectroscopy, and stereochemistry. Laboratory is required and will i ...
Section D19: Alkanes, Alkenes and Alcohols
... 3.4 recall the products of the complete and incomplete combustion of alkanes 3.5 describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light. 3.6 recall that alkenes have the general formula CnH2n 3.7 draw displayed formulae for alkenes with up to four ...
... 3.4 recall the products of the complete and incomplete combustion of alkanes 3.5 describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light. 3.6 recall that alkenes have the general formula CnH2n 3.7 draw displayed formulae for alkenes with up to four ...
Aldehydes and Ketones
... ketone with a phosphonium ylide- a compound with a negatively-charged carbon bonded to a positively-charged phosphorus atom. The result is the replacement of the carbonyl oxygen with the carbon group on the ylide. We will learn how to prepare this Wittig reagent (the ylide), and how it reacts with t ...
... ketone with a phosphonium ylide- a compound with a negatively-charged carbon bonded to a positively-charged phosphorus atom. The result is the replacement of the carbonyl oxygen with the carbon group on the ylide. We will learn how to prepare this Wittig reagent (the ylide), and how it reacts with t ...
Aldehydes and Ketones
... site of electrophilic attack. Since ordinary carbanions (R: −) and hydride ions (H: −) are very poor leaving groups (unlike halide ions, X−) nucleophilic substitution does not usually occur at the carbonyl carbon of aldehydes or ketones. ...
... site of electrophilic attack. Since ordinary carbanions (R: −) and hydride ions (H: −) are very poor leaving groups (unlike halide ions, X−) nucleophilic substitution does not usually occur at the carbonyl carbon of aldehydes or ketones. ...
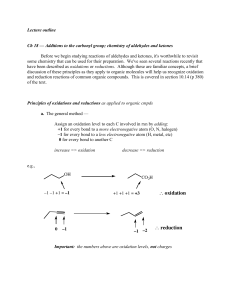
Wed March 3 lecture
... Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or reductions. Although these are familiar concepts, a brief discussion of these ...
... Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or reductions. Although these are familiar concepts, a brief discussion of these ...
Synthetic Transformations of C=O Compounds Reaction Summary
... (i.e. R-Li + H-O-H à R-H + LiOH) • Organolithium Reagents (R-Li) o Preparation: R-X + 2 Li à R-Li + LiX o React with aldehydes and ketones to provide 2o and 3o alcohols, respectively. O ...
... (i.e. R-Li + H-O-H à R-H + LiOH) • Organolithium Reagents (R-Li) o Preparation: R-X + 2 Li à R-Li + LiX o React with aldehydes and ketones to provide 2o and 3o alcohols, respectively. O ...
Chapter 20 reactions of carbonyls
... • Aldehydes can also be oxidized selectively in the presence of other functional groups using silver(I) oxide in aqueous ammonium hydroxide (Tollen’s reagent). • Since ketones have no H on the carbonyl carbon, they do not undergo this oxidation reaction. ...
... • Aldehydes can also be oxidized selectively in the presence of other functional groups using silver(I) oxide in aqueous ammonium hydroxide (Tollen’s reagent). • Since ketones have no H on the carbonyl carbon, they do not undergo this oxidation reaction. ...
Elias James Corey

Elias James ""E.J."" Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry ""for his development of the theory and methodology of organic synthesis"", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.