Alcohols
... borohydride, but dangerous to work with. • Reduces ketones and aldehydes into the corresponding alcohol. • Converts esters and carboxylic acids to 1º alcohols. Chapter 10 ...
... borohydride, but dangerous to work with. • Reduces ketones and aldehydes into the corresponding alcohol. • Converts esters and carboxylic acids to 1º alcohols. Chapter 10 ...
Organic Chemistry Lecture Outline Carbonyl
... substitution reactions. Carbonyl groups bonded to large, bulky substituents (eg., tertiary carbons) react slower with nucleophiles than carbonyl groups with smaller, less bulky substituents. Electronic Effects Electronic effects influence the reactivity of the electrophilic carbonyl carbon in nucleo ...
... substitution reactions. Carbonyl groups bonded to large, bulky substituents (eg., tertiary carbons) react slower with nucleophiles than carbonyl groups with smaller, less bulky substituents. Electronic Effects Electronic effects influence the reactivity of the electrophilic carbonyl carbon in nucleo ...
167KB - NZQA
... Carboxylic acid (butanoic acid) is obtained by reacting a mixture of butan-1ol with acidified potassium dichromate solution (under reflux conditions) until all of the reactant has been converted to butanoic acid. Observations: orange Cr2O72– to green /, purple MnO4– to colourless / aldehyde condense ...
... Carboxylic acid (butanoic acid) is obtained by reacting a mixture of butan-1ol with acidified potassium dichromate solution (under reflux conditions) until all of the reactant has been converted to butanoic acid. Observations: orange Cr2O72– to green /, purple MnO4– to colourless / aldehyde condense ...
NCEA Level 3 Chemistry (91391) 2013
... Carboxylic acid (butanoic acid) is obtained by reacting a mixture of butan-1ol with acidified potassium dichromate solution (under reflux conditions) until all of the reactant has been converted to butanoic acid. Observations: orange Cr2O72– to green /, purple MnO4– to colourless / aldehyde condense ...
... Carboxylic acid (butanoic acid) is obtained by reacting a mixture of butan-1ol with acidified potassium dichromate solution (under reflux conditions) until all of the reactant has been converted to butanoic acid. Observations: orange Cr2O72– to green /, purple MnO4– to colourless / aldehyde condense ...
CHAPTER II. A Facile Synthesis of Arylacetic Acid Derivatives via
... Only a limited number of synthetic methods are available in the literature for the synthesis of α-hydroxy arylacetic acid (mandelic acid) and derivatives. The present work deals about an intramolecular cannizzaro reaction, can be effectively catalyzed by In(OTf)3 producing α-hydroxy arylacetic acids ...
... Only a limited number of synthetic methods are available in the literature for the synthesis of α-hydroxy arylacetic acid (mandelic acid) and derivatives. The present work deals about an intramolecular cannizzaro reaction, can be effectively catalyzed by In(OTf)3 producing α-hydroxy arylacetic acids ...
Stereoselective reactions of the carbonyl group
... • Underrated chiral auxiliary - easily introduced, performs many reactions & can be readily removed • One auxiliary can give either enantiomer of product • Selectivity dependent on whether reagent can chelate two groups Advanced organic ...
... • Underrated chiral auxiliary - easily introduced, performs many reactions & can be readily removed • One auxiliary can give either enantiomer of product • Selectivity dependent on whether reagent can chelate two groups Advanced organic ...
Exam 1 Review Sheet Chapter 15 Chemistry 110b
... Acetals and ketals and their hemi- forms. Be able to identify these functional groups. Know the mechanism of formation/hydrolysis for an acetal (e.g., acetaldehyde + 2CH3OH + H+(cat.)). Be aware of the utility of acetals and ketals [and their thio (sulfur) analogs] as protecting groups in organic sy ...
... Acetals and ketals and their hemi- forms. Be able to identify these functional groups. Know the mechanism of formation/hydrolysis for an acetal (e.g., acetaldehyde + 2CH3OH + H+(cat.)). Be aware of the utility of acetals and ketals [and their thio (sulfur) analogs] as protecting groups in organic sy ...
6.5. alcohols
... The optimum temperature for fermentation is around 38oC At lower temperatures the rate of reaction is too slow. At higher temperatures the yeast dies and the enzymes denature. Fermentation is done in an absence of air because the presence of air can cause extra reactions to occur. Air oxidises the e ...
... The optimum temperature for fermentation is around 38oC At lower temperatures the rate of reaction is too slow. At higher temperatures the yeast dies and the enzymes denature. Fermentation is done in an absence of air because the presence of air can cause extra reactions to occur. Air oxidises the e ...
13_lecture_ppt
... • Both aldehydes and ketones are readily reduced to alcohols – Reduction occurs with hydrogen as the reducing agent ...
... • Both aldehydes and ketones are readily reduced to alcohols – Reduction occurs with hydrogen as the reducing agent ...
Ketones and Aldehydes Reading: Wade chapter 18, sections 18
... Irreversible additions to carbonyls: Strong Nucleophiles 1. Grignard and organolithium addition: Highly basic grignard and organolithium reagents add to carbonyl irreversibly to give alcohols; aldehydes give 2° alcohols and ketones give 3° alcohols; formaldehyde gives a 1° alcohol: ...
... Irreversible additions to carbonyls: Strong Nucleophiles 1. Grignard and organolithium addition: Highly basic grignard and organolithium reagents add to carbonyl irreversibly to give alcohols; aldehydes give 2° alcohols and ketones give 3° alcohols; formaldehyde gives a 1° alcohol: ...
Carbohydrates
... 1. Ketoses (Sugars with Ketone groups) vs Aldoses (Sugars with Aldehyde groups) 2. Fischer projections a. Aldose D family: triose – tetrose - pentose – hexose (Figure 23-3) b. Erythro and Threo: History as hydroxyl groups on same or opposite side of Fischer projection in tetroses (Section 23-4) c. D ...
... 1. Ketoses (Sugars with Ketone groups) vs Aldoses (Sugars with Aldehyde groups) 2. Fischer projections a. Aldose D family: triose – tetrose - pentose – hexose (Figure 23-3) b. Erythro and Threo: History as hydroxyl groups on same or opposite side of Fischer projection in tetroses (Section 23-4) c. D ...
File - Garbally Chemistry
... 3. No molecular hydrogen produced – hence no hydrogen free radicals have been formed. For the propagation steps 1. Thousands of chloromethane molecules are produced for every one photon of light used. This suggests a chain reaction consistent with theproposed mechanism. 2. No molecular hydrogen prod ...
... 3. No molecular hydrogen produced – hence no hydrogen free radicals have been formed. For the propagation steps 1. Thousands of chloromethane molecules are produced for every one photon of light used. This suggests a chain reaction consistent with theproposed mechanism. 2. No molecular hydrogen prod ...
Carolina Aguirre, Rosa Arrieta, Soledad Anjarí, Andrés Illanes
... exhibited seems to berelated to a direct solvent effect rather than to reduced water activity, since the enzyme has proven to be active at very low water activity in solid-phase systems (23). However, water-miscible organic cosolvents are a suitable medium to perform the synthesis of β-lactam antibi ...
... exhibited seems to berelated to a direct solvent effect rather than to reduced water activity, since the enzyme has proven to be active at very low water activity in solid-phase systems (23). However, water-miscible organic cosolvents are a suitable medium to perform the synthesis of β-lactam antibi ...
Enantioselective Henry Reactions under Dual Lewis Acid/Amine
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
(a) Draw a primary, a secondary, and a tertiary alcohol for the
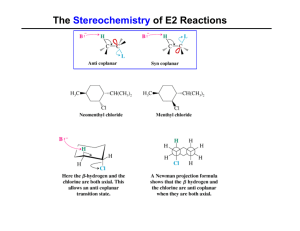
... C is the major product and D is the minor product. There are 2 possible products because when the double bond is broken, an H (or –OH) will bond to one C (and a –OH group (or H) will bond with the other C). The product will depend on which (C) the H (or the –OH) bond to. ...
... C is the major product and D is the minor product. There are 2 possible products because when the double bond is broken, an H (or –OH) will bond to one C (and a –OH group (or H) will bond with the other C). The product will depend on which (C) the H (or the –OH) bond to. ...
Chapter 3 Properties of organic compounds
... substitution of alcohols is increased by heating the reaction mixture under reflux – this way the reaction mixture can be heated for a period of time without material (reactant, product or solvent) evaporating and being lost from the flask. Oxidation – using acidified KMnO4 or acidified K2Cr2O7. Pri ...
... substitution of alcohols is increased by heating the reaction mixture under reflux – this way the reaction mixture can be heated for a period of time without material (reactant, product or solvent) evaporating and being lost from the flask. Oxidation – using acidified KMnO4 or acidified K2Cr2O7. Pri ...
Unit 8 Homework Packet
... 26. Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst. Xe(g) + 2F2(g) → XeF4(s) ...
... 26. Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst. Xe(g) + 2F2(g) → XeF4(s) ...
Chapter 15
... • 4) Polyalkylations often occur – When putting activating groups on, in general, such as alkyl groups, the product after the first addition is more reactive than the starting material – Example – Polyacylations do not occur since the acyl group is a deactivating group ...
... • 4) Polyalkylations often occur – When putting activating groups on, in general, such as alkyl groups, the product after the first addition is more reactive than the starting material – Example – Polyacylations do not occur since the acyl group is a deactivating group ...
Pyrrolidine-2-carboxylic Acid (l
... This feature focuses on a reagent chosen by a postgraduate, highlighting the uses and preparation of the reagent in current research ...
... This feature focuses on a reagent chosen by a postgraduate, highlighting the uses and preparation of the reagent in current research ...
Programme
... and related compounds is documented in this Thesis. Their photobehaviour in aqueous solvent media varied dramatically from their well-known behaviour in organic solvents and suggests unique and unprecedented mechanistic pathways. The aqueous photoredox chemistry of various substituted benzophenones ...
... and related compounds is documented in this Thesis. Their photobehaviour in aqueous solvent media varied dramatically from their well-known behaviour in organic solvents and suggests unique and unprecedented mechanistic pathways. The aqueous photoredox chemistry of various substituted benzophenones ...
Electrochemical oxidation of cinnamic acid using stainless steel
... The IR spectrum of the product shows absorption peaks with reduced intensity and this is characteristic of the IR spectra taken using film technique. The IR spectrum of the product has absorption frequencies corresponding to aromatic stretching (2910 cm-~), aroamtic substituted alkene (1640 and 1630 ...
... The IR spectrum of the product shows absorption peaks with reduced intensity and this is characteristic of the IR spectra taken using film technique. The IR spectrum of the product has absorption frequencies corresponding to aromatic stretching (2910 cm-~), aroamtic substituted alkene (1640 and 1630 ...
Addition Reactions of Carbonyls Part 1
... Unlike the molecules on the previous page, acetals and ketals are stable in base. They won’t collapse to carbonyl groups except in the presence of acid and water. This is very useful… ...
... Unlike the molecules on the previous page, acetals and ketals are stable in base. They won’t collapse to carbonyl groups except in the presence of acid and water. This is very useful… ...
Wolff–Kishner reduction

The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. Originally reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912, it has been applied to the total synthesis of scopadulcic acid B, aspidospermidine and dysidiolide.In general, the reaction mechanism first involves the in situ generation of a hydrazone by condensation of hydrazine with the ketone or aldehyde substrate. Sometimes it is however advantageous to use a pre-formed hydrazone as substrate (see modifications). The hydrazone is deprotonated by alkoxide base followed by a concerted, rate-determining step in which a diimide anion is formed. Collapse of this alkyldiimde with loss of N2 leads to formation of an alkylanion which can be protonated by solvent to give the desired product.Because the Wolff–Kishner reduction requires highly basic conditions, it is unsuitable for base-sensitive substrates. However, this method can be superior over the related Clemmensen reduction for acid-sensitive compounds such as pyrroles and for high-molecular weight compounds.