Experiment 7: Acidity of Alcohols Williamson Ether Synthesis of
... In separate, dry test tubes, place 2 mL each of methanol, ethanol, 2-propanol (isopropyl alcohol) and 2-methyl-2-propanol. Using tweezers, add a small (ca. 2 mm across) piece of sodium to each test tube. Record all observations in your notebook. What do you observe? What gas is formed from the react ...
... In separate, dry test tubes, place 2 mL each of methanol, ethanol, 2-propanol (isopropyl alcohol) and 2-methyl-2-propanol. Using tweezers, add a small (ca. 2 mm across) piece of sodium to each test tube. Record all observations in your notebook. What do you observe? What gas is formed from the react ...
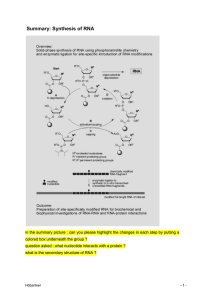
Synthesis of RNA - Stamm revision
... TOM group compared to TBDMS during internucleotide bond formation allows for high coupling yields in short coupling times. Data for RNA synthesis in high yield and high quality have been reported for oligonucleotides up to 84 nucleotides in length. The 5’-O-silyl-2’-O-ACE phosphoramidites 3 were des ...
... TOM group compared to TBDMS during internucleotide bond formation allows for high coupling yields in short coupling times. Data for RNA synthesis in high yield and high quality have been reported for oligonucleotides up to 84 nucleotides in length. The 5’-O-silyl-2’-O-ACE phosphoramidites 3 were des ...
Working with Hazardous Chemicals
... as nucleophilic catalysts in group transfer reactions.5 The esterification proceeds without the need of a preformed, activated carboxylic acid derivative, at room temperature, under nonacidic, mildly basic conditions. In addition to dichloromethane other aprotic solvents of comparable polarity such ...
... as nucleophilic catalysts in group transfer reactions.5 The esterification proceeds without the need of a preformed, activated carboxylic acid derivative, at room temperature, under nonacidic, mildly basic conditions. In addition to dichloromethane other aprotic solvents of comparable polarity such ...
Properties of , -Unsaturated Aldehydes and Ketones
... ,-Unsaturated aldehydes and ketones undergo the reactions typical of their component functional groups. The conjugated carbonyl group of ,-unsaturated aldehydes and ketones can undergo reactions involving the entire functional system by: ...
... ,-Unsaturated aldehydes and ketones undergo the reactions typical of their component functional groups. The conjugated carbonyl group of ,-unsaturated aldehydes and ketones can undergo reactions involving the entire functional system by: ...
Project Overview
... Since Grignard reagents are powerful nucleophiles, we cannot prepare a Grignard reagent from any organic halide that contains a carbonyl, epoxy, nitro, or cyano (–CN) group Ch. 12 - 62 ...
... Since Grignard reagents are powerful nucleophiles, we cannot prepare a Grignard reagent from any organic halide that contains a carbonyl, epoxy, nitro, or cyano (–CN) group Ch. 12 - 62 ...
PART 3 Principles and Applications of Organometallics in Catalysis
... The effect of a catalyst is to change the rate of conversion of a substrate into products, but they do not change the position of an equilibrium. The thermodynamics of the reaction concerned have to be favourable at the outset; catalysts can't perform the miracle of pushing a reaction up the thermod ...
... The effect of a catalyst is to change the rate of conversion of a substrate into products, but they do not change the position of an equilibrium. The thermodynamics of the reaction concerned have to be favourable at the outset; catalysts can't perform the miracle of pushing a reaction up the thermod ...
Alkene - Synthesis
... E1 Dehydrohalogenation 2° or 3° alkyl halides requires a good ionizing solvent: alcohol or water. no strong nucleophile or base Rearrangements can occur. ...
... E1 Dehydrohalogenation 2° or 3° alkyl halides requires a good ionizing solvent: alcohol or water. no strong nucleophile or base Rearrangements can occur. ...
A Straightforward Route to Enantiopure Pyrrolizidines and
... homogeneous catalysts for the production of oxygenates [26-33], including methanol, ethanol, and especially ethylene glycol. The principal shortcoming of all homogeneous CO hydrogenation reactions is their low catalytic activity, which results in the need to use high catalyst loadings and drastic re ...
... homogeneous catalysts for the production of oxygenates [26-33], including methanol, ethanol, and especially ethylene glycol. The principal shortcoming of all homogeneous CO hydrogenation reactions is their low catalytic activity, which results in the need to use high catalyst loadings and drastic re ...
Excercises 6-10
... the rate constants kf, kr and the equilibrium constant K in thermodynamic equilibrium. 3. Draw the reaction scheme (including intermediate) and the reaction profile of a nucleophilic substitution via the ...
... the rate constants kf, kr and the equilibrium constant K in thermodynamic equilibrium. 3. Draw the reaction scheme (including intermediate) and the reaction profile of a nucleophilic substitution via the ...
Ethers and Epoxides
... Tetrahydrofuran (THF) is a solvent that is a cyclic ether Epoxides contain a C-O-C unit which make-up a three membered ring Thiols (R–S–H) and sulfides (R–S–R) are sulfur (for oxygen) analogs of alcohols and ethers ...
... Tetrahydrofuran (THF) is a solvent that is a cyclic ether Epoxides contain a C-O-C unit which make-up a three membered ring Thiols (R–S–H) and sulfides (R–S–R) are sulfur (for oxygen) analogs of alcohols and ethers ...
Iodoform Test - organicchem.org
... The iodoform reagent is a mixture of iodine (I2) and potassium iodide (KI). Reaction of a methylketone with strong base promotes the formation of an enolate which reacts with the electrophilic I2 to generate an -iodomethylketone. Addition of two more equivalents of base and I2 lead to formation of ...
... The iodoform reagent is a mixture of iodine (I2) and potassium iodide (KI). Reaction of a methylketone with strong base promotes the formation of an enolate which reacts with the electrophilic I2 to generate an -iodomethylketone. Addition of two more equivalents of base and I2 lead to formation of ...
Demonstrate skill in organic chemistry techniques.
... Predict reactions of alkenes. Learning Objectives Demonstrate understanding of electrophilic additions. Predict products of reactions of alkenes, including regiochemistry and stereochemistry. Explain observed products of alkene reactions using a mechanistic approach. Solve multistep synthesis proble ...
... Predict reactions of alkenes. Learning Objectives Demonstrate understanding of electrophilic additions. Predict products of reactions of alkenes, including regiochemistry and stereochemistry. Explain observed products of alkene reactions using a mechanistic approach. Solve multistep synthesis proble ...
Procedure - organicchem.org
... The iodoform reagent is a mixture of iodine (I 2) and potassium iodide (KI). Reaction of a methylketone with strong base promotes the formation of an enolate which reacts with the electrophilic I2 to generate an -iodomethylketone. Addition of two more equivalents of base and I2 lead to formation of ...
... The iodoform reagent is a mixture of iodine (I 2) and potassium iodide (KI). Reaction of a methylketone with strong base promotes the formation of an enolate which reacts with the electrophilic I2 to generate an -iodomethylketone. Addition of two more equivalents of base and I2 lead to formation of ...
11. 5-member heterocycles with 1 and heteroatoms
... The (1,2) and (3,4) bonds can also be formed from N-substituted αaminoketones and formamide and heat. The product will be a 1,4disubstituted imidazole, but here since R=R1=Hydrogen, imidazole itself is the product. The yield of this reaction is moderate, but it seems to be the most effective method ...
... The (1,2) and (3,4) bonds can also be formed from N-substituted αaminoketones and formamide and heat. The product will be a 1,4disubstituted imidazole, but here since R=R1=Hydrogen, imidazole itself is the product. The yield of this reaction is moderate, but it seems to be the most effective method ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the
... The parent chain is numbered to give the ketone carbonyl the lowest possible number In common nomenclature simple ketones are named by preceding the word ketone with the names of both groups attached to the ketone carbonyl ...
... The parent chain is numbered to give the ketone carbonyl the lowest possible number In common nomenclature simple ketones are named by preceding the word ketone with the names of both groups attached to the ketone carbonyl ...
Summary of Reactions Which Will Appear on Exams
... 42. REACTIONS OF GRIGNARD REAGENTS AND ORGANOLITHIUM COMPOUNDS WITH ESTERS TO FORM TERTIARY ALCOHOLS ...
... 42. REACTIONS OF GRIGNARD REAGENTS AND ORGANOLITHIUM COMPOUNDS WITH ESTERS TO FORM TERTIARY ALCOHOLS ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.