Reactions of carbohydrates
... There are four D-aldopentoses. Draw Fischer projections of each of them. Then draw Fischer projections of the aldaric acids they would yield. Label each center as a R or S configuration. Circle the aldaric acids that are optically inactive? ...
... There are four D-aldopentoses. Draw Fischer projections of each of them. Then draw Fischer projections of the aldaric acids they would yield. Label each center as a R or S configuration. Circle the aldaric acids that are optically inactive? ...
Chapter 12 Alcohols, Phenols, Ethers, Aldehydes, and Ketones
... Write the equations for the reaction of 2-propanol when it ...
... Write the equations for the reaction of 2-propanol when it ...
SCH 4U
... b. Explain how you could use physical properties to tell the substances apart – be sure to be specific as to what would be observed. (3 marks) ...
... b. Explain how you could use physical properties to tell the substances apart – be sure to be specific as to what would be observed. (3 marks) ...
Hydrocarbons and Functional Groups
... 3. Name the branches based on how many carbons they have, and at what carbon they branch off of. 2-methyl-3-ethylheptane* *this is NOT the final name of this molecule 5. Put the branch names in alphabetical order. 3-ethyl-2methylheptane ...
... 3. Name the branches based on how many carbons they have, and at what carbon they branch off of. 2-methyl-3-ethylheptane* *this is NOT the final name of this molecule 5. Put the branch names in alphabetical order. 3-ethyl-2methylheptane ...
Week - Syllabus | Chaminade
... career interests, the multi-dimensionality of this discipline can be demonstrated much more easily. It is hoped that at the end of the semester, the student will be fulfilled intellectually, in addition to accumulating the knowledge, which is essential for his/her studies in molecular and/or biomedi ...
... career interests, the multi-dimensionality of this discipline can be demonstrated much more easily. It is hoped that at the end of the semester, the student will be fulfilled intellectually, in addition to accumulating the knowledge, which is essential for his/her studies in molecular and/or biomedi ...
Qualitative Analysis
... - To better understand several chemical reactions. - To identify an unknown chemical by testing its chemical and physical properties. Introduction: In this experiment, you will investigate solubility behavior and some typical reactions of carboxylic acids, amines, aromatic compounds, alkenes, alcoho ...
... - To better understand several chemical reactions. - To identify an unknown chemical by testing its chemical and physical properties. Introduction: In this experiment, you will investigate solubility behavior and some typical reactions of carboxylic acids, amines, aromatic compounds, alkenes, alcoho ...
organic compounds in three dimensions
... Structural Isomers: Two or more compounds with the same molecular formula but different arrangements of atoms are called isomers. Isomers differ in one or more physical or chemical properties such as boiling point, color, solubility, reactivity, and density. Several different types of isomerism are ...
... Structural Isomers: Two or more compounds with the same molecular formula but different arrangements of atoms are called isomers. Isomers differ in one or more physical or chemical properties such as boiling point, color, solubility, reactivity, and density. Several different types of isomerism are ...
1.2 The Chemicals of Life - Father Michael McGivney
... Hydrolysis Reaction Two subunits break apart through the addition of a water molecule. Hydration synthesis is a catabolic reaction that releases energy. ...
... Hydrolysis Reaction Two subunits break apart through the addition of a water molecule. Hydration synthesis is a catabolic reaction that releases energy. ...
Laboratory and Demonstrations Evaporation and Intermolecular
... then measure, the temperature change for several other liquids. You will encounter two types of organic compounds in this experiment — alkanes and alcohols. The two alkanes are pentane, C5H12, and hexane, C6H14. In addition to carbon and hydrogen atoms, alcohols also contain the -OH functional group ...
... then measure, the temperature change for several other liquids. You will encounter two types of organic compounds in this experiment — alkanes and alcohols. The two alkanes are pentane, C5H12, and hexane, C6H14. In addition to carbon and hydrogen atoms, alcohols also contain the -OH functional group ...
Chapter 17: Aldehydes and Ketones: Nucleophilic Addition to the
... Spectra of Aldehydes and Ketones: The 1H chemical shift range for the aldehyde proton is δ 9-10 ppm The aldehyde proton will couple to the protons on the α-carbon with a typical coupling constant of J ≈ 2 Hz A carbonyl will slightly deshield the protons on the α-carbon; ...
... Spectra of Aldehydes and Ketones: The 1H chemical shift range for the aldehyde proton is δ 9-10 ppm The aldehyde proton will couple to the protons on the α-carbon with a typical coupling constant of J ≈ 2 Hz A carbonyl will slightly deshield the protons on the α-carbon; ...
Chapter 11 Carboxylic Anhydrides, Esters, and Amides
... In principle, we can form an amide by treating a carboxylic acid with an amine and removing -OH from the acid and an -H from the amine. • In practice what occurs if the two are mixed is an acid-base reaction to form an ammonium salt. • If this salt is heated to a high enough temperature, water is el ...
... In principle, we can form an amide by treating a carboxylic acid with an amine and removing -OH from the acid and an -H from the amine. • In practice what occurs if the two are mixed is an acid-base reaction to form an ammonium salt. • If this salt is heated to a high enough temperature, water is el ...
Organic Reactions Worksheet
... 11. The complete and incomplete combustion of 2-pentanol (incomplete will have both CO and C in addition to carbon dioxide). How would the flame colors differ? ...
... 11. The complete and incomplete combustion of 2-pentanol (incomplete will have both CO and C in addition to carbon dioxide). How would the flame colors differ? ...
Organic Chemistry Fifth Edition
... Anion of salt is in a relatively unsolvated state in benzene (sometimes referred to as a "naked anion") Unsolvated anion is very reactive Only catalytic quantities of 18-crown-6 are needed KF ...
... Anion of salt is in a relatively unsolvated state in benzene (sometimes referred to as a "naked anion") Unsolvated anion is very reactive Only catalytic quantities of 18-crown-6 are needed KF ...
Reaction with Hydrogen Halides
... Copyright © 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education. ...
... Copyright © 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education. ...
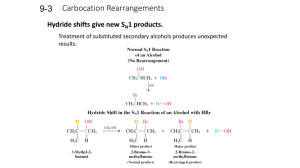
Carbocation Rearrangements
... Other carbocation rearrangements are due to alkyl shifts. Alkyl shifts, rather than hydride shifts, can occur when a carbocation lacks a suitable secondary or tertiary hydrogen next to the positively charged carbon. ...
... Other carbocation rearrangements are due to alkyl shifts. Alkyl shifts, rather than hydride shifts, can occur when a carbocation lacks a suitable secondary or tertiary hydrogen next to the positively charged carbon. ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.