Exam 2
... • All written responses must be in English. At the end of the examination • Place the answer sheet for multiple-choice questions inside the front cover of this book. Students are NOT permitted to bring mobile phones and/or any other unauthorised electronic devices into the examination room. ...
... • All written responses must be in English. At the end of the examination • Place the answer sheet for multiple-choice questions inside the front cover of this book. Students are NOT permitted to bring mobile phones and/or any other unauthorised electronic devices into the examination room. ...
Kayla Martin
... atoms that hold hydrogen atoms. The formulas found in CH3OH (methanol) C2h5OH (ethanol), and CH3CH2CHOHCH3 (2 butanol). Alcoholic beverages such as beer and wine, that have ethyl alcohol (ethanol) were made before history was recorded. Methanol, the first member of the alcohol family has a boiling p ...
... atoms that hold hydrogen atoms. The formulas found in CH3OH (methanol) C2h5OH (ethanol), and CH3CH2CHOHCH3 (2 butanol). Alcoholic beverages such as beer and wine, that have ethyl alcohol (ethanol) were made before history was recorded. Methanol, the first member of the alcohol family has a boiling p ...
Polyvalent Iodine in Synthesis (more than just Dess
... of 1, found to be explosive, had 43.5% iodine by elemental analysis (calculated 45.32% for 1) although none of the samples of 1 prepared by our method had any unexpected decrease in the percentage of iodine. They also had some bromine (4%) in 1 after washing with only water. We washed with water and ...
... of 1, found to be explosive, had 43.5% iodine by elemental analysis (calculated 45.32% for 1) although none of the samples of 1 prepared by our method had any unexpected decrease in the percentage of iodine. They also had some bromine (4%) in 1 after washing with only water. We washed with water and ...
Taj Brands
... Flash point : 8 °C Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, wherein one H atom is replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas ...
... Flash point : 8 °C Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, wherein one H atom is replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas ...
Unit 9 Chemical Equations and Reactions Balancing Equations Notes
... Single Replacement- a metal will _________________ a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Double Replacement- the metals in ionic compounds _________________ places. Combustion- an ____________________ compound containing carbon, hydrogen and some ...
... Single Replacement- a metal will _________________ a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Double Replacement- the metals in ionic compounds _________________ places. Combustion- an ____________________ compound containing carbon, hydrogen and some ...
program
... Domain C: Carbon chemistry Subdomain C2: Other applications of carbon bonds Subdomain C3: The candidate can indicate for a number of carbon bonds which types of reactions they can undergo and which products are then formed. The candidate can: ...
... Domain C: Carbon chemistry Subdomain C2: Other applications of carbon bonds Subdomain C3: The candidate can indicate for a number of carbon bonds which types of reactions they can undergo and which products are then formed. The candidate can: ...
9.1-10.5 Organic Chemistry
... Evaluation: Determine if your prediction was verified or falsified. Was the predictive power of the concept of London forces judged to be acceptable based on the evidence? Was the purpose of the ...
... Evaluation: Determine if your prediction was verified or falsified. Was the predictive power of the concept of London forces judged to be acceptable based on the evidence? Was the purpose of the ...
Press here to hemy 102 lab manual
... 2- Write the symbols for the atoms to show which atoms are attached to which, and connect them with a single bond (a dash, representing two electrons). Chemical formulas are often written in the order in which the atoms are connected to the molecule or ion, as in HCN. When a central atom has a group ...
... 2- Write the symbols for the atoms to show which atoms are attached to which, and connect them with a single bond (a dash, representing two electrons). Chemical formulas are often written in the order in which the atoms are connected to the molecule or ion, as in HCN. When a central atom has a group ...
Liquid-gas phase-boundary catalytic system
... simulation theory. The use of TiO2 as inorganic precursor and organic surfactant, however, has not been reported. In our recent report [5 ], well-aligned titanium dioxide was successfully synthesized by sol-gel method by using tetra-n-butyl orthotitanate (TBOT) as titanium dioxide precursor. Wellali ...
... simulation theory. The use of TiO2 as inorganic precursor and organic surfactant, however, has not been reported. In our recent report [5 ], well-aligned titanium dioxide was successfully synthesized by sol-gel method by using tetra-n-butyl orthotitanate (TBOT) as titanium dioxide precursor. Wellali ...
1. What is the best definition of rate of reaction? A. The time it takes
... Alex’s hypothesis was that the rate will be affected by changing the concentrations of the propanone and the iodine, as the reaction can happen without a catalyst. Hannah’s hypothesis was that as the catalyst is involved in the reaction, the concentrations of the propanone, iodine and the hydrogen i ...
... Alex’s hypothesis was that the rate will be affected by changing the concentrations of the propanone and the iodine, as the reaction can happen without a catalyst. Hannah’s hypothesis was that as the catalyst is involved in the reaction, the concentrations of the propanone, iodine and the hydrogen i ...
Experiment 1
... 2- Write the symbols for the atoms to show which atoms are attached to which, and connect them with a single bond (a dash, representing two electrons). Chemical formulas are often written in the order in which the atoms are connected to the molecule or ion, as in HCN. When a central atom has a group ...
... 2- Write the symbols for the atoms to show which atoms are attached to which, and connect them with a single bond (a dash, representing two electrons). Chemical formulas are often written in the order in which the atoms are connected to the molecule or ion, as in HCN. When a central atom has a group ...
polymerisation
... Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensation polymers Recall that peptides are examples of condensation polymers ...
... Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensation polymers Recall that peptides are examples of condensation polymers ...
19_01_05.html
... in water, many stearate ions cluster together to form spherical aggregates; carboxylate ions on the outside and nonpolar tails on the inside ...
... in water, many stearate ions cluster together to form spherical aggregates; carboxylate ions on the outside and nonpolar tails on the inside ...
No Slide Title
... Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensation polymers Recall that peptides are examples of condensation polymers ...
... Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensation polymers Recall that peptides are examples of condensation polymers ...
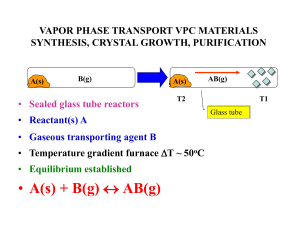
vapor phase transport vpc materials synthesis, crystal growth
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
examination paper - University of Calgary
... The compound A, C8H12, reacted with Br2/CHCl3 to give a colourless solution. Reaction of A with aq. H2SO4 gave B, C8H16O2, as a mixture of two stereoisomers, both of which reacted rapidly with the Lucas reagent (HCl / ZnCl2). Subsequent reaction of either of the stereoisomers of B by heating with co ...
... The compound A, C8H12, reacted with Br2/CHCl3 to give a colourless solution. Reaction of A with aq. H2SO4 gave B, C8H16O2, as a mixture of two stereoisomers, both of which reacted rapidly with the Lucas reagent (HCl / ZnCl2). Subsequent reaction of either of the stereoisomers of B by heating with co ...
105
... In section 10.2, you learned that a redox reaction involves changes in oxidation numbers. If an element undergoes oxidation, its oxidation number increases. If an element undergoes reduction, its oxidation number decreases. When balancing equations by the half-reaction method in section 10.3, you so ...
... In section 10.2, you learned that a redox reaction involves changes in oxidation numbers. If an element undergoes oxidation, its oxidation number increases. If an element undergoes reduction, its oxidation number decreases. When balancing equations by the half-reaction method in section 10.3, you so ...
Chapter 1--Title
... Esters Synthesis of Esters: Esterification Acid catalyzed reaction of alcohols and carboxylic acids to form esters is called Fischer esterification Fischer esterification is an equilibrium process ...
... Esters Synthesis of Esters: Esterification Acid catalyzed reaction of alcohols and carboxylic acids to form esters is called Fischer esterification Fischer esterification is an equilibrium process ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.