Chapter 4
... Often called a neutralization reaction Because the acid neutralizes the base. Often titrate to determine concentrations. Solution of known concentration (titrant), is added to the unknown (analyte), until the equivalence point is reached where enough titrant has been added to ...
... Often called a neutralization reaction Because the acid neutralizes the base. Often titrate to determine concentrations. Solution of known concentration (titrant), is added to the unknown (analyte), until the equivalence point is reached where enough titrant has been added to ...
Chemistry - Textbooks Online
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
введение в общую introductio to the general ch ведение в общую
... part of a substance that has the physical and chemical properties of that substance. Some elements exist in form of molecules. For example, hydrogen and oxygen exist as two-atom molecules. Sulfur may exist as an eight-atom molecule, S8, while phosphorus may exist as a four-atom molecule, P 4. Other ...
... part of a substance that has the physical and chemical properties of that substance. Some elements exist in form of molecules. For example, hydrogen and oxygen exist as two-atom molecules. Sulfur may exist as an eight-atom molecule, S8, while phosphorus may exist as a four-atom molecule, P 4. Other ...
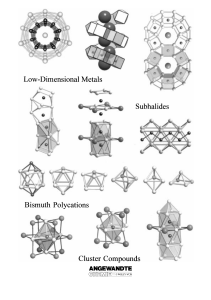
From the Metal to the Molecule
... Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research m ...
... Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research m ...
Chemical Equations
... 4Fe(s) + 3O2 → 2Fe2O3 2KClO3(s) → 2KCl(s) + 3O2(g) 2Mg + O2(g) →2MgO 3Ca(OH)2(s) + 2H3PO4(aq) → Ca3(PO4)2(s) +6H2O(l) P4(s) +5O2 (g) →P4O10(s) Ba(ClO3)2(aq) + H2SO4(aq) → 2HClO3(aq) + BaSO4(s) 4NH3(g)+5O2(g) →4NO(g) + 6H2O(g) C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) 2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2 ...
... 4Fe(s) + 3O2 → 2Fe2O3 2KClO3(s) → 2KCl(s) + 3O2(g) 2Mg + O2(g) →2MgO 3Ca(OH)2(s) + 2H3PO4(aq) → Ca3(PO4)2(s) +6H2O(l) P4(s) +5O2 (g) →P4O10(s) Ba(ClO3)2(aq) + H2SO4(aq) → 2HClO3(aq) + BaSO4(s) 4NH3(g)+5O2(g) →4NO(g) + 6H2O(g) C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) 2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2 ...
SCH4U - Unit 1
... Have you ever seen a picture of the first computer? Figure 1.1 below depicts what the first computer looked like. It may seem odd and funny to look back at such pictures, but most technologies are constant works in progress. Computers now come in tiny devices such as phones and laptops. The developm ...
... Have you ever seen a picture of the first computer? Figure 1.1 below depicts what the first computer looked like. It may seem odd and funny to look back at such pictures, but most technologies are constant works in progress. Computers now come in tiny devices such as phones and laptops. The developm ...
Chemistry MCQs - Target Publications
... With the change in educational curriculum it’s now time for a change in Competitive Examinations. NEET and ISEET are all poised to take over the decade old MHT-CET. The change is obvious not merely in the names but also at the competitive levels. The state level entrance examination is ushered aside ...
... With the change in educational curriculum it’s now time for a change in Competitive Examinations. NEET and ISEET are all poised to take over the decade old MHT-CET. The change is obvious not merely in the names but also at the competitive levels. The state level entrance examination is ushered aside ...
Chapter 4: Aqueous Reactions and Solution Stoichiometry
... the reaction between aqueous solutions of acetic acid and barium hydroxide. Then write the net ionic equation. Write a balanced molecular equation for the reaction between aqueous solutions of carbonic acid and potassium hydroxide. Then write the net ionic ...
... the reaction between aqueous solutions of acetic acid and barium hydroxide. Then write the net ionic equation. Write a balanced molecular equation for the reaction between aqueous solutions of carbonic acid and potassium hydroxide. Then write the net ionic ...
Document
... 5. Halogens: The oxidation number of fluorine is -1. Each of the other halogens (Cl, Br, I) has an oxidation number of -1 in binary compounds, except when the other element is another halogen above it in the periodic table or the other element is oxygen. 6. Compounds and ions: The sum of the oxidat ...
... 5. Halogens: The oxidation number of fluorine is -1. Each of the other halogens (Cl, Br, I) has an oxidation number of -1 in binary compounds, except when the other element is another halogen above it in the periodic table or the other element is oxygen. 6. Compounds and ions: The sum of the oxidat ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... 1. For each of the redox equations below: deduce the oxidation and reduction half equations deduce the oxidizing and reducing agent (a) Mg (s) + Cl2 (g) MgCl2 (s) (b) 2NaCl (aq) + F2 (aq) 2NaF (aq) + CI2 (aq) (c) CuSO4 (aq) + Zn (s) ZnSO4 (aq) + Cu (s) (d) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2 (a ...
... 1. For each of the redox equations below: deduce the oxidation and reduction half equations deduce the oxidizing and reducing agent (a) Mg (s) + Cl2 (g) MgCl2 (s) (b) 2NaCl (aq) + F2 (aq) 2NaF (aq) + CI2 (aq) (c) CuSO4 (aq) + Zn (s) ZnSO4 (aq) + Cu (s) (d) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2 (a ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... 1. For each of the redox equations below: deduce the oxidation and reduction half equations deduce the oxidizing and reducing agent (a) Mg (s) + Cl2 (g) MgCl2 (s) (b) 2NaCl (aq) + F2 (aq) 2NaF (aq) + CI2 (aq) (c) CuSO4 (aq) + Zn (s) ZnSO4 (aq) + Cu (s) (d) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2 (a ...
... 1. For each of the redox equations below: deduce the oxidation and reduction half equations deduce the oxidizing and reducing agent (a) Mg (s) + Cl2 (g) MgCl2 (s) (b) 2NaCl (aq) + F2 (aq) 2NaF (aq) + CI2 (aq) (c) CuSO4 (aq) + Zn (s) ZnSO4 (aq) + Cu (s) (d) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2 (a ...
NCERT Solution - Mywayteaching
... to its high electronegativity. It also exhibits the oxidation state of −1 (H2O2), zero (O2), and +2 (OF2). However, the stability of the −2 oxidation state decreases on moving down a group due to a decrease in the electronegativity of the elements. The heavier ...
... to its high electronegativity. It also exhibits the oxidation state of −1 (H2O2), zero (O2), and +2 (OF2). However, the stability of the −2 oxidation state decreases on moving down a group due to a decrease in the electronegativity of the elements. The heavier ...
BSPH 111 - Refresher Chemistry
... elements in the periodic table is classified according to its atomic number, which is the number of protons in that element's nucleus. Protons have a charge of +1, electrons have a charge of -1, and neutrons have no charge. Neutral atoms have the same number of electrons and protons, but they can ha ...
... elements in the periodic table is classified according to its atomic number, which is the number of protons in that element's nucleus. Protons have a charge of +1, electrons have a charge of -1, and neutrons have no charge. Neutral atoms have the same number of electrons and protons, but they can ha ...
Astrochemistry and Star Formation
... Ruffle & Herbst 2000, 2001). Unfortunately, the chemistry that occurs on grain surfaces and physical gas-grain interactions are still poorly understood, and represent perhaps the major challenge mentioned in the title of this paper. Other challenges remaining to be solved by astrochemists include a ...
... Ruffle & Herbst 2000, 2001). Unfortunately, the chemistry that occurs on grain surfaces and physical gas-grain interactions are still poorly understood, and represent perhaps the major challenge mentioned in the title of this paper. Other challenges remaining to be solved by astrochemists include a ...
Single Replacement Reactions - Tri
... • Single Replacement Reactions occur when one element replaces another in a compound. • A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). • element + compound compound + element A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) ...
... • Single Replacement Reactions occur when one element replaces another in a compound. • A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). • element + compound compound + element A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.