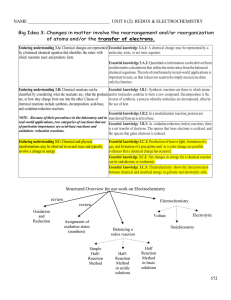
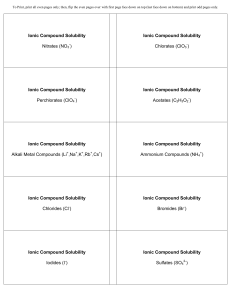
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Chemistry booklet
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
O 2 - Montville.net
... This means a 12 ounce bottle of water would have 19.7 “moles” of water…a much easier-to-work-with Adopted from "Chemistry You number! Need to Know" by Kelly Deters ...
... This means a 12 ounce bottle of water would have 19.7 “moles” of water…a much easier-to-work-with Adopted from "Chemistry You number! Need to Know" by Kelly Deters ...
Chemistry and Biochemistry
... beginning of each semester (fall, spring, summer). For consideration for a graduate assistantship, all application materials should be received at least five months prior to the desired starting date. Students whose undergraduate degree is not equivalent to the American Chemical Society certified Ba ...
... beginning of each semester (fall, spring, summer). For consideration for a graduate assistantship, all application materials should be received at least five months prior to the desired starting date. Students whose undergraduate degree is not equivalent to the American Chemical Society certified Ba ...
File
... 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hydrides) 38. NaH is an example of __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hyd ...
... 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hydrides) 38. NaH is an example of __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hyd ...
CHAPTER 20 METALLURGY AND THE CHEMISTRY OF METALS
... Both Li and Mg form oxides (Li2O and MgO). Other Group 1A metals (Na, K, etc.) also form peroxides and superoxides. In Group 1A, only Li forms nitride (Li3N), like Mg (Mg3N2). Li resembles Mg in that its carbonate, fluoride, and phosphate have low solubilities. ...
... Both Li and Mg form oxides (Li2O and MgO). Other Group 1A metals (Na, K, etc.) also form peroxides and superoxides. In Group 1A, only Li forms nitride (Li3N), like Mg (Mg3N2). Li resembles Mg in that its carbonate, fluoride, and phosphate have low solubilities. ...
university of zagreb - Hrvatsko fizikalno društvo
... other natural sciences, biomedicine and technology very much. Recently the physics methods are successfully applied in other fields such as economy and sociology. Chemistry is the basis for understanding of the processes in all sorts of matter including living beings, at the atomic/molecular level. ...
... other natural sciences, biomedicine and technology very much. Recently the physics methods are successfully applied in other fields such as economy and sociology. Chemistry is the basis for understanding of the processes in all sorts of matter including living beings, at the atomic/molecular level. ...
Organic Chemistry Curriculum Map - Belle Vernon Area School District
... Standard: 3.2.C.A4 – Predict how combinations of substances can result in physical and/or chemical changes. Anchor: CHEM.B.1.1 – Explain how the mole is a fundamental unit of chemistry. Eligible Content CHEM.B.1.1.1 – Apply the mole concept to representative particles (e.g., counting, determining ...
... Standard: 3.2.C.A4 – Predict how combinations of substances can result in physical and/or chemical changes. Anchor: CHEM.B.1.1 – Explain how the mole is a fundamental unit of chemistry. Eligible Content CHEM.B.1.1.1 – Apply the mole concept to representative particles (e.g., counting, determining ...
OCR Gateway Science
... (a) A solution of concentration 0.1 mol/dm3 that contains 1 mol of sodium chloride. (b) A solution of concentration 0.5 mol/dm3 that contains 0.1 mol of sodium nitrate. (c) A solution of concentration 0.1 mol/dm3 that contains 0.25 mol of copper sulfate. ...
... (a) A solution of concentration 0.1 mol/dm3 that contains 1 mol of sodium chloride. (b) A solution of concentration 0.5 mol/dm3 that contains 0.1 mol of sodium nitrate. (c) A solution of concentration 0.1 mol/dm3 that contains 0.25 mol of copper sulfate. ...
Chemistry, Biology
... and sub-atomic level, is built on knowledge of these classical theories and concepts. Students should think of physics in terms of scales. Whereas the classical theories such as Newton’s laws of motion apply to common physical systems that are larger than the size of atoms, a more comprehensive theo ...
... and sub-atomic level, is built on knowledge of these classical theories and concepts. Students should think of physics in terms of scales. Whereas the classical theories such as Newton’s laws of motion apply to common physical systems that are larger than the size of atoms, a more comprehensive theo ...
Physics, Chemistry
... and sub-atomic level, is built on knowledge of these classical theories and concepts. Students should think of physics in terms of scales. Whereas the classical theories such as Newton’s laws of motion apply to common physical systems that are larger than the size of atoms, a more comprehensive theo ...
... and sub-atomic level, is built on knowledge of these classical theories and concepts. Students should think of physics in terms of scales. Whereas the classical theories such as Newton’s laws of motion apply to common physical systems that are larger than the size of atoms, a more comprehensive theo ...
Chemistry XII - Kendriya Vidyalaya IIM,Lucknow
... Where z is electrochemical equivalent. Unit of electrochemical equivalent is gram/coulomb Faraday is charge on 1 mole of electrons. ...
... Where z is electrochemical equivalent. Unit of electrochemical equivalent is gram/coulomb Faraday is charge on 1 mole of electrons. ...
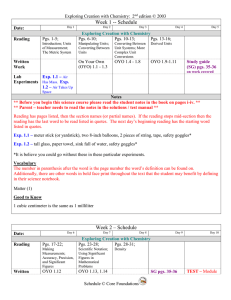
Week 1 -- Schedule
... ** Before you begin this science course please read the student notes in the book on pages i-iv. ** ** Parent – teacher needs to read the notes in the solutions / test manual ** Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has ...
... ** Before you begin this science course please read the student notes in the book on pages i-iv. ** ** Parent – teacher needs to read the notes in the solutions / test manual ** Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has ...
Class-XII, Summer assignment
... 1. Though nitrogen exhibits +5 oxidation state, it does not form pentahalide. Give reason. Ans: Nitrogen valence electronic configuration ns2np3 due to absence of empty d- orbitals, it can not extend its valence to 5. 2. PH3 has lower boiling point than NH3. Why? Ans: Unlike NH3, PH3 molecules are n ...
... 1. Though nitrogen exhibits +5 oxidation state, it does not form pentahalide. Give reason. Ans: Nitrogen valence electronic configuration ns2np3 due to absence of empty d- orbitals, it can not extend its valence to 5. 2. PH3 has lower boiling point than NH3. Why? Ans: Unlike NH3, PH3 molecules are n ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... • This reaction gives the predicted product, but you had better carry it out in the hood, or you will be very unpopular! • But just as in the previous examples, a gas is formed as a product of this reaction. Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Aqueous Reactions © 2009, Prentice-Hall, Inc ...
... • This reaction gives the predicted product, but you had better carry it out in the hood, or you will be very unpopular! • But just as in the previous examples, a gas is formed as a product of this reaction. Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Aqueous Reactions © 2009, Prentice-Hall, Inc ...
Chapter Four - Salina USD 305
... • Write formulas for the reactants and predicted products for the chemical reactions that follow: 1. Solid calcium carbonate is strongly heated in a test tube. ...
... • Write formulas for the reactants and predicted products for the chemical reactions that follow: 1. Solid calcium carbonate is strongly heated in a test tube. ...
Chemical Reactions
... same number of particles. • Moles are numbers of particles • You can treat reactions as if they happen liters at a time, as long as you keep the temperature and pressure the same. ...
... same number of particles. • Moles are numbers of particles • You can treat reactions as if they happen liters at a time, as long as you keep the temperature and pressure the same. ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.