I- Introduction
... molecule or crystalline compounds is required or confirmation of the presence or position of certain organic functional groups is sought. Such examinations are described as structural analysis and they may be considered as more detailed forms of analysis. Any species that are the subjects of either ...
... molecule or crystalline compounds is required or confirmation of the presence or position of certain organic functional groups is sought. Such examinations are described as structural analysis and they may be considered as more detailed forms of analysis. Any species that are the subjects of either ...
Second Year - WordPress.com
... The carbonates and phosphates of which elements are insoluble in water ...
... The carbonates and phosphates of which elements are insoluble in water ...
Chapter 15
... compounds are highly soluble and dissolve easily in water. Water molecules attract each of the ions of an ionic compound and pull the ions away from one another. • The solution that forms when an ionic compound dissolves in water can conduct an electric current because the ions are charged and are a ...
... compounds are highly soluble and dissolve easily in water. Water molecules attract each of the ions of an ionic compound and pull the ions away from one another. • The solution that forms when an ionic compound dissolves in water can conduct an electric current because the ions are charged and are a ...
Improved Synthesis of Seven-Coordinate Molybdenum( I I) and
... [W(CN-t-C4H&I]I. A solution of 0.234 g (0.92 "01) of iodine dissolved in 10 mL of methanol was added dropwise with stirring to a solution containing 0.484 g (0.94 mmol) of W(CN-tc4&)&0)3 dissolved in 25 mL of methanol. After the addition was complete, 0.26 g (3.1 "01) of tert-butyl isocyanidewas add ...
... [W(CN-t-C4H&I]I. A solution of 0.234 g (0.92 "01) of iodine dissolved in 10 mL of methanol was added dropwise with stirring to a solution containing 0.484 g (0.94 mmol) of W(CN-tc4&)&0)3 dissolved in 25 mL of methanol. After the addition was complete, 0.26 g (3.1 "01) of tert-butyl isocyanidewas add ...
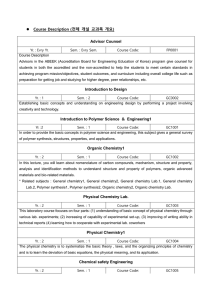
Course Description Word File
... Sem. : 2 Course Code: GC1022 To increase the design ability of polymer materials by studying the fundamentals of polymer materials and characterization, by selecting polymer materials, which can be found in our living environment and industries, and performing team projects with them in order to lea ...
... Sem. : 2 Course Code: GC1022 To increase the design ability of polymer materials by studying the fundamentals of polymer materials and characterization, by selecting polymer materials, which can be found in our living environment and industries, and performing team projects with them in order to lea ...
Chapter 4 Student Presentation
... • Reaction of sulfides with acid gives rise to H2S(g). • FeS (s) + 2 HCl (aq) FeCl2 (aq) + H2S (g) • Reaction of sulfites with acid gives rise to SO2 (g). • SrSO3 (s) + 2 HI (aq) SrI2 (aq) + SO2 (g) + H2O (l) ...
... • Reaction of sulfides with acid gives rise to H2S(g). • FeS (s) + 2 HCl (aq) FeCl2 (aq) + H2S (g) • Reaction of sulfites with acid gives rise to SO2 (g). • SrSO3 (s) + 2 HI (aq) SrI2 (aq) + SO2 (g) + H2O (l) ...
Stoichiometry of Ozonation of Environmentally
... double bonds could potentially result in 2:1 DB:O3 stoichiometry. However, carbonyl oxides do not undergo cycloaddition to double bonds unless the latter are strongly polarized by substituent groups (15, 16). None of the reactants used in this work have such activated double bonds. Furthermore, rece ...
... double bonds could potentially result in 2:1 DB:O3 stoichiometry. However, carbonyl oxides do not undergo cycloaddition to double bonds unless the latter are strongly polarized by substituent groups (15, 16). None of the reactants used in this work have such activated double bonds. Furthermore, rece ...
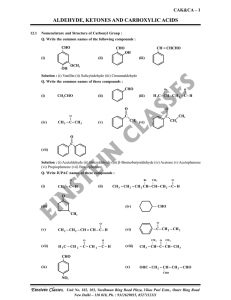
aldehyde, ketones and carboxylic acids
... Solution : A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermed ...
... Solution : A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermed ...
Chapter 4 – Part 1
... Define solid, liquid, and gas Know the Kinetic Molecular Theory and how particles behave in different states of matter Know qualitative and quantitative observations Know the difference between homogeneous and heterogeneous mixtures Know the difference between elements and atoms Know the ...
... Define solid, liquid, and gas Know the Kinetic Molecular Theory and how particles behave in different states of matter Know qualitative and quantitative observations Know the difference between homogeneous and heterogeneous mixtures Know the difference between elements and atoms Know the ...
DOE Chemistry 1
... Details the principles of ion exchange in the context of water purity. Discusses typical water treatment methods and the basis for these methods. Module 5 - Hazards of Chemicals and Gases Explains why certain chemicals are considered hazardous to facility personnel. Includes general safety rules on ...
... Details the principles of ion exchange in the context of water purity. Discusses typical water treatment methods and the basis for these methods. Module 5 - Hazards of Chemicals and Gases Explains why certain chemicals are considered hazardous to facility personnel. Includes general safety rules on ...
TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
... When making solution A, 25 cm3 of the NaOH solution reacts with 25 cm3 of the CH3COOH solution. This forms some ethanoate ions, CH3COO-(aq), and leaves some unreacted ethanoic acid molecules, CH3COOH. CH3COOH(aq) + OH−(aq) → CH3COO−(aq) + H2O(l) So, solution A contains a mixture of a weak acid, CH3C ...
... When making solution A, 25 cm3 of the NaOH solution reacts with 25 cm3 of the CH3COOH solution. This forms some ethanoate ions, CH3COO-(aq), and leaves some unreacted ethanoic acid molecules, CH3COOH. CH3COOH(aq) + OH−(aq) → CH3COO−(aq) + H2O(l) So, solution A contains a mixture of a weak acid, CH3C ...
Integrated Physics and Chemistry
... of state; Describe the laws of conservation of mass and conservation of energy, and explain how they apply to changes of state Perform calculations involving density; Apply the laws of conservation of mass and conservation of energy to chemical and physical changes; Evaluate materials and their prop ...
... of state; Describe the laws of conservation of mass and conservation of energy, and explain how they apply to changes of state Perform calculations involving density; Apply the laws of conservation of mass and conservation of energy to chemical and physical changes; Evaluate materials and their prop ...
aq - Haverford Alchemy
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
Cyanuric Acid and Cyanurates
... amino groups. The dissociation constants of melamine in aqueous solutions were found to be K1 = 1.26 × 10–9; K2 = 1.58 × 10–14, and K3 = 1 × 10–17 [63]. Although melamine is a weak base, it nevertheless can form salts [53, 63–70]. However, it almost always acts as a monoacidic base. The yellow needl ...
... amino groups. The dissociation constants of melamine in aqueous solutions were found to be K1 = 1.26 × 10–9; K2 = 1.58 × 10–14, and K3 = 1 × 10–17 [63]. Although melamine is a weak base, it nevertheless can form salts [53, 63–70]. However, it almost always acts as a monoacidic base. The yellow needl ...
w_4-3 Chemistry of Nitrogen Compounds
... Since nitrogen trichloride is fairly volatile (similar to chloroform), it will tend to volatilize into the atmosphere, thereby reducing the concentration in the water. This will be most pronounced in spas with their higher temperatures and use of aeration. Nitrogen trichloride is also decomposed by ...
... Since nitrogen trichloride is fairly volatile (similar to chloroform), it will tend to volatilize into the atmosphere, thereby reducing the concentration in the water. This will be most pronounced in spas with their higher temperatures and use of aeration. Nitrogen trichloride is also decomposed by ...
Chapter 1 Chirality in clinical analysis 1.1. Introduction
... More than 250 organic acids and glycine conjugates are either typically present or may possibly encountered in urine [13]. More than 65 inherited metabolic abnormalities are known to produce a characteristic urinary organic acid pattern, essential for diagnosis and follow-up [13-16]. Possible origin ...
... More than 250 organic acids and glycine conjugates are either typically present or may possibly encountered in urine [13]. More than 65 inherited metabolic abnormalities are known to produce a characteristic urinary organic acid pattern, essential for diagnosis and follow-up [13-16]. Possible origin ...
Chapter 23 Metals and Metallurgy
... Metallurgy The science and technology of extracting metals from their natural sources and preparing them for ...
... Metallurgy The science and technology of extracting metals from their natural sources and preparing them for ...
Chapter 2 The Components of Matter
... 3. For Type II metals with only two common oxidation states an older, Latin system was once used; while it is not employed very often it is useful to know some simple rules regarding it. It is sometimes called the “-ous/-ic” system, where the LOWER charged cation will be denoted by the latin root na ...
... 3. For Type II metals with only two common oxidation states an older, Latin system was once used; while it is not employed very often it is useful to know some simple rules regarding it. It is sometimes called the “-ous/-ic” system, where the LOWER charged cation will be denoted by the latin root na ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.