Module 3 Transition elements - Pearson Schools and FE Colleges
... wavelengths of light that have not been absorbed. For example, a solution of copper(II) sulfate appears pale blue because the solution absorbs the red/orange region of the electromagnetic spectrum and reflects or transmits the blue. Many coloured inorganic compounds contain transition metal ions. In ...
... wavelengths of light that have not been absorbed. For example, a solution of copper(II) sulfate appears pale blue because the solution absorbs the red/orange region of the electromagnetic spectrum and reflects or transmits the blue. Many coloured inorganic compounds contain transition metal ions. In ...
Exam 980617 - NTOU-Chem
... C) [CrNO2 (CN)5 ]2D) [Co (en)2 ClBr ]+ E) [Pt (NH3 ) Cl3 ]Answer: A 5) A concentration cell consists of two Ni / Ni2+ electrodes. The electrolyte in half-cell A is 0.50 M and the electrolyte in half-cell B is 0.0025 M. Which combination below correctly pairs the anode and the cell voltage? A) A, + 0 ...
... C) [CrNO2 (CN)5 ]2D) [Co (en)2 ClBr ]+ E) [Pt (NH3 ) Cl3 ]Answer: A 5) A concentration cell consists of two Ni / Ni2+ electrodes. The electrolyte in half-cell A is 0.50 M and the electrolyte in half-cell B is 0.0025 M. Which combination below correctly pairs the anode and the cell voltage? A) A, + 0 ...
Dimethylsulphoxide Complexes of Vanadium(III) o
... further evidence ofoxygen-bonded DMSO ligands. A similar band·splitting has also been reported for [Fe)DMSO).J" where Berney and Weber (1968) have suggested from their studies of several metalDMSO complexes that all the v(M-Q) bands are in fact split since the observed bands are broad but only in th ...
... further evidence ofoxygen-bonded DMSO ligands. A similar band·splitting has also been reported for [Fe)DMSO).J" where Berney and Weber (1968) have suggested from their studies of several metalDMSO complexes that all the v(M-Q) bands are in fact split since the observed bands are broad but only in th ...
Magnetic study of the electronic states of B-DNA and M
... Two different grades of salmon-DNA 共a kind of B-DNA兲 were purchased from Wako Pure Chemical Industries, Ltd.: DNA powder 共S-powder兲 and DNA fiber 共S-fiber兲. The S-fiber is a purified form of S-powder, thus the fibrous morphology might reflect the rodlike linear structure of the DNA double helix. Met ...
... Two different grades of salmon-DNA 共a kind of B-DNA兲 were purchased from Wako Pure Chemical Industries, Ltd.: DNA powder 共S-powder兲 and DNA fiber 共S-fiber兲. The S-fiber is a purified form of S-powder, thus the fibrous morphology might reflect the rodlike linear structure of the DNA double helix. Met ...
Chapter 2. Atoms, Molecules, and Ion
... Naming Compounds IV. Acids Oxoacids have the same central atom but a different number of O atoms. Addition of one O atom “ – ic” acid “per … – ic” acid HClO3 ...
... Naming Compounds IV. Acids Oxoacids have the same central atom but a different number of O atoms. Addition of one O atom “ – ic” acid “per … – ic” acid HClO3 ...
Novel Functionalized Rare Earth Metal Clusters and Polymers
... in europium(III) compounds which varies its wavelength notably upon changes of ligands and site symmetry.[15] The 4f-electrons in Ln3+-ions are capable of undergoing 4f-4f-tranisitions which are Laporte forbidden, i.e. the sum of the angular momenta of the electrons in the initial and final state do ...
... in europium(III) compounds which varies its wavelength notably upon changes of ligands and site symmetry.[15] The 4f-electrons in Ln3+-ions are capable of undergoing 4f-4f-tranisitions which are Laporte forbidden, i.e. the sum of the angular momenta of the electrons in the initial and final state do ...
BS5-Ch 2.
... If the metal forms only one positive ion, the cation name is the English name for the metal If the metal forms more than one positive ion, the cation name is the English name followed, without a space, by the numerical value of the charge written as a Roman numeral in parentheses ...
... If the metal forms only one positive ion, the cation name is the English name for the metal If the metal forms more than one positive ion, the cation name is the English name followed, without a space, by the numerical value of the charge written as a Roman numeral in parentheses ...
Types of Compounds
... • Because ionic compounds are electrically neutral, one can determine the formula of a compound this way: The charge on the cation becomes the subscript on the anion. The charge on the anion becomes the subscript on the cation. If these subscripts are not in the lowest wholenumber ratio, divid ...
... • Because ionic compounds are electrically neutral, one can determine the formula of a compound this way: The charge on the cation becomes the subscript on the anion. The charge on the anion becomes the subscript on the cation. If these subscripts are not in the lowest wholenumber ratio, divid ...
Chapter 2: Atoms, Molecules, and Ions
... C) Ernest Rutherford D) William Thomson E) John Dalton 17. Alpha particles beamed at thin metal foil may A) pass directly through without changing direction B) be slightly diverted by attraction to electrons C) be reflected by direct contact with nuclei D) A and C E) A, B, and C 18. Which one of th ...
... C) Ernest Rutherford D) William Thomson E) John Dalton 17. Alpha particles beamed at thin metal foil may A) pass directly through without changing direction B) be slightly diverted by attraction to electrons C) be reflected by direct contact with nuclei D) A and C E) A, B, and C 18. Which one of th ...
A P P E N D I C E S
... excitations from the highest filled molecular orbital to the lower energy unfilled molecular orbitals. For many organic molecules (e.g. benzene) these transitions lie in the UV-region of the spectrum. However for transition metal complex ions such transitions often fall in the visible region of the ...
... excitations from the highest filled molecular orbital to the lower energy unfilled molecular orbitals. For many organic molecules (e.g. benzene) these transitions lie in the UV-region of the spectrum. However for transition metal complex ions such transitions often fall in the visible region of the ...
Functional group migrations between boron and
... scorpionate ligands to sting. Originally, the sting was considered as the coordination of the third ‘‘arm’’ of the ligand to a metal centre. However, this perception was altered when it was discovered that the ‘‘hydride species’’ from the borohydride unit could be transferred to the metal centre (i. ...
... scorpionate ligands to sting. Originally, the sting was considered as the coordination of the third ‘‘arm’’ of the ligand to a metal centre. However, this perception was altered when it was discovered that the ‘‘hydride species’’ from the borohydride unit could be transferred to the metal centre (i. ...
Chapter 7 Ionic and Metallic Bonding
... inner (core) electrons Put one dot for each valence electron (8 maximum) They don’t pair up until they have to (Hund’s rule) ...
... inner (core) electrons Put one dot for each valence electron (8 maximum) They don’t pair up until they have to (Hund’s rule) ...
Experiment 4 - Chemistry| |UCC
... Soap scum is formed when the Ca2+ ion binds with the soap. This causes an insoluble compound that precipitates to form the scum you see. Soap actually softens hard water by removing the Ca2+ ions from the water When hard water is heated, CaCO3 precipitates out, which then clogs pipes and industrial ...
... Soap scum is formed when the Ca2+ ion binds with the soap. This causes an insoluble compound that precipitates to form the scum you see. Soap actually softens hard water by removing the Ca2+ ions from the water When hard water is heated, CaCO3 precipitates out, which then clogs pipes and industrial ...
Study of η6 - cyclic π-perimeter hydrocarbon ruthenium complexes
... complexes have emerged as versatile intermediates in organic synthesis as a consequence of the ease with which the arene ligand can be functionalized. 1,2 The applications of half-sandwich η6 -arene ruthenium complexes are extensive, particularly in synthetic organic chemistry. These purely inorgani ...
... complexes have emerged as versatile intermediates in organic synthesis as a consequence of the ease with which the arene ligand can be functionalized. 1,2 The applications of half-sandwich η6 -arene ruthenium complexes are extensive, particularly in synthetic organic chemistry. These purely inorgani ...
Introduction to Computational Chemistry
... Computational chemists are hired by academic institutions, government agencies, and all types of industries. The pharmaceutical industry in particular has embraced computational chemistry as an effective tool in the design of new drugs. Computational chemists are employed to study all types of chemi ...
... Computational chemists are hired by academic institutions, government agencies, and all types of industries. The pharmaceutical industry in particular has embraced computational chemistry as an effective tool in the design of new drugs. Computational chemists are employed to study all types of chemi ...
Atoms and Materials for Engineering
... connected to at least one other atom. The connections are made by atomic bonds. There are three important kinds of primary atomic bonds: 1) ionic 2) covalent 3) metallic. To really understand each kind, you would need to read many pages of explanation. So let us just try for some simple descriptions ...
... connected to at least one other atom. The connections are made by atomic bonds. There are three important kinds of primary atomic bonds: 1) ionic 2) covalent 3) metallic. To really understand each kind, you would need to read many pages of explanation. So let us just try for some simple descriptions ...
1 O 2
... Structure and function of hemoglobin III. The oxygen saturation of hemoglobin is characterised by cooperativity: the extent of saturation changes in a ratio of 1:4:24:9 with an increase of the number of bound oxygen molecules . Reason: The size of the porphyrin ring in case of FeII complex is not l ...
... Structure and function of hemoglobin III. The oxygen saturation of hemoglobin is characterised by cooperativity: the extent of saturation changes in a ratio of 1:4:24:9 with an increase of the number of bound oxygen molecules . Reason: The size of the porphyrin ring in case of FeII complex is not l ...
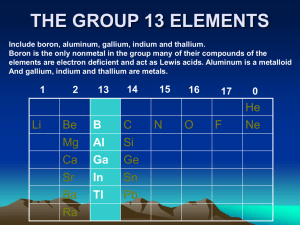
THE GROUP 13 ELEMENTS - University of the Witwatersrand
... called sapphire, ruby, emerald or amethyst depending o the amount of metal ion impurities. Dehydration of Al(OH)3 at temperatures below 900 °C result in the formation of γ-alumina, which is metastable polycrystalline form with a defect spinel structure. The α and γ forms of Ga2O3 have the same struc ...
... called sapphire, ruby, emerald or amethyst depending o the amount of metal ion impurities. Dehydration of Al(OH)3 at temperatures below 900 °C result in the formation of γ-alumina, which is metastable polycrystalline form with a defect spinel structure. The α and γ forms of Ga2O3 have the same struc ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.