Sorption
... • Outer Sphere surface complex ion remains bounded to the hydration shell so it does not bind directly to the surface, attraction is purely electrostatic • Inner Sphere surface complex ion bonds to a specific site on the surface, this ignores overall electrostatic interaction with bulk surface ( ...
... • Outer Sphere surface complex ion remains bounded to the hydration shell so it does not bind directly to the surface, attraction is purely electrostatic • Inner Sphere surface complex ion bonds to a specific site on the surface, this ignores overall electrostatic interaction with bulk surface ( ...
Sorption
... • Outer Sphere surface complex ion remains bounded to the hydration shell so it does not bind directly to the surface, attraction is purely electrostatic • Inner Sphere surface complex ion bonds to a specific site on the surface, this ignores overall electrostatic interaction with bulk surface ( ...
... • Outer Sphere surface complex ion remains bounded to the hydration shell so it does not bind directly to the surface, attraction is purely electrostatic • Inner Sphere surface complex ion bonds to a specific site on the surface, this ignores overall electrostatic interaction with bulk surface ( ...
(Lecture(21) - MSU Chemistry
... 1. be'familiar'with'the'properties'of'and'bonding'in'the'different'allotropes'of'carbon.' 2. know'the'different'types'of'compounds'formed'by'carbon'and'be'able'to'classify' carbon'compounds.' 3. be'able'to'determine'the'oxidation'number'of'C'in'carbon'compounds.' 4. know'the'relationship'between'hyb ...
... 1. be'familiar'with'the'properties'of'and'bonding'in'the'different'allotropes'of'carbon.' 2. know'the'different'types'of'compounds'formed'by'carbon'and'be'able'to'classify' carbon'compounds.' 3. be'able'to'determine'the'oxidation'number'of'C'in'carbon'compounds.' 4. know'the'relationship'between'hyb ...
Experiment 13
... Complex ions. In aqueous solution, metal cations do not exist as free ions. Instead, they form complex ions with surrounding water molecules. In most hydrated cations, either two, four, or six water molecules are loosely bonded to a central metal cation. Copper ion has a coordination number of 4, so ...
... Complex ions. In aqueous solution, metal cations do not exist as free ions. Instead, they form complex ions with surrounding water molecules. In most hydrated cations, either two, four, or six water molecules are loosely bonded to a central metal cation. Copper ion has a coordination number of 4, so ...
Photochemistry of tetrasulfido complexes of molybdenum (VI
... The irradiation of aqueous solutions of [WS412-led to results that were very similar to those obtained for [MoS4I2-. In the presence of air the photolysis of [WS4I2- was accompanied by spectral changes (Figure 2) that indicated clearly the formation of [W02S2]2-.4i*42 At higher complex concentration ...
... The irradiation of aqueous solutions of [WS412-led to results that were very similar to those obtained for [MoS4I2-. In the presence of air the photolysis of [WS4I2- was accompanied by spectral changes (Figure 2) that indicated clearly the formation of [W02S2]2-.4i*42 At higher complex concentration ...
Sn19.3Cu4.7As22I8: a New Clathrate-I
... semiconducting clathrates are surveyed in a recent review.9 Clathrates with the type I structure based on the group 14 elements trigger growing interest because of the expectations that thermoelectric materials of a new generation will emerge following the guidelines of the “Phonon Glass, Electron C ...
... semiconducting clathrates are surveyed in a recent review.9 Clathrates with the type I structure based on the group 14 elements trigger growing interest because of the expectations that thermoelectric materials of a new generation will emerge following the guidelines of the “Phonon Glass, Electron C ...
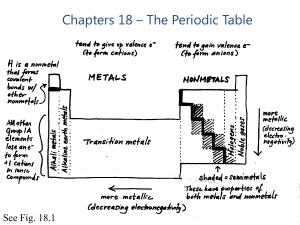
Chapters 18 – The Periodic Table
... 3. Sulfur trioxide (SO3) and sulfuric acid (H2SO4). SO3, formed from SO2 over V2O5 catalysts, is then converted to sulfuric acid. Sulfuric acid is the cheapest strong acid and is so widely used in industry that its production level is an indicator of a nation’s economic strength. Strong dehydrating ...
... 3. Sulfur trioxide (SO3) and sulfuric acid (H2SO4). SO3, formed from SO2 over V2O5 catalysts, is then converted to sulfuric acid. Sulfuric acid is the cheapest strong acid and is so widely used in industry that its production level is an indicator of a nation’s economic strength. Strong dehydrating ...
Slide 1
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
Synthesis, characterization and spectroscopic investigation
... energy transfer to the Tm3 + ion. The emission intensities in the tetrakis C[Tm(acac)4] complexes are 20 times stronger than those in the tris [Tm(acac)3(H2O)3] complex (Fig. 5). This increase in luminescence intensity is due to the hindrance from water molecules in the first coordination sphere in t ...
... energy transfer to the Tm3 + ion. The emission intensities in the tetrakis C[Tm(acac)4] complexes are 20 times stronger than those in the tris [Tm(acac)3(H2O)3] complex (Fig. 5). This increase in luminescence intensity is due to the hindrance from water molecules in the first coordination sphere in t ...
Second Semester Notes 09-10
... Redox reaction – a reaction in which electrons are transferred from one atom to another; charge is conserved Oxidation – loss of electrons from atoms of a substance; oxidation # increases; substance that is oxidized acts as the REDUCING ...
... Redox reaction – a reaction in which electrons are transferred from one atom to another; charge is conserved Oxidation – loss of electrons from atoms of a substance; oxidation # increases; substance that is oxidized acts as the REDUCING ...
Chlorine K-Edge X-ray Absorption Spectroscopy
... metal centers such as Mn, Fe, Ni, Cu, and Mo. Recently, it has been demonstrated that the absorption edges of ligands such as S and Cl can be used to study the electronic structure of first transition series metal complexes2-4 and copper-containing metallo proteins.5 Earlier studies by DeRose found ...
... metal centers such as Mn, Fe, Ni, Cu, and Mo. Recently, it has been demonstrated that the absorption edges of ligands such as S and Cl can be used to study the electronic structure of first transition series metal complexes2-4 and copper-containing metallo proteins.5 Earlier studies by DeRose found ...
Chapter 8. Chiral Catalysts
... Figure 2.1. However, the variations in such distal positions may produce important differences in the conformational preferences of the chiral ligand and hence of the chiral complex, the corresponding reaction intermediate and the diastereomeric transition states. Those conformational variations hav ...
... Figure 2.1. However, the variations in such distal positions may produce important differences in the conformational preferences of the chiral ligand and hence of the chiral complex, the corresponding reaction intermediate and the diastereomeric transition states. Those conformational variations hav ...
Limiting Reactant WS with Answers
... 8) The amino acid arginine is an essential component of all proteins. This compound contains 41.36% carbon, 8.10% hydrogen, and 32.17% nitrogen, with the remainder being oxygen. a) Determine the empirical formula of arginine. b) The molar mass of arginine is known to be between 100 and 200 g/mol. D ...
... 8) The amino acid arginine is an essential component of all proteins. This compound contains 41.36% carbon, 8.10% hydrogen, and 32.17% nitrogen, with the remainder being oxygen. a) Determine the empirical formula of arginine. b) The molar mass of arginine is known to be between 100 and 200 g/mol. D ...
Common Oxidizing Agents
... solutions of lithium hydroxide and hydrobromic acid are mixed. How does the size of the bromide ion compare to the chloride ion on the periodic table? ans: OH- + H+→ H2O. Br- is bigger than Cl-. ...
... solutions of lithium hydroxide and hydrobromic acid are mixed. How does the size of the bromide ion compare to the chloride ion on the periodic table? ans: OH- + H+→ H2O. Br- is bigger than Cl-. ...
Atomic combinations: Electronegativity and ionic
... 2 Ionic Bonding 2.1 The nature of the ionic bond You will remember that when atoms bond, electrons are either shared or they are transferred between the atoms that are bonding. In covalent bonding, electrons are shared between the atoms. There is another type of bonding, where electrons are transfer ...
... 2 Ionic Bonding 2.1 The nature of the ionic bond You will remember that when atoms bond, electrons are either shared or they are transferred between the atoms that are bonding. In covalent bonding, electrons are shared between the atoms. There is another type of bonding, where electrons are transfer ...
Sample Paper Chemistry - Educomp Solutions Ltd.
... (i) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. (ii) There are two -NH2 groups in semicarbazide. However, only one is in volved in the formation of semicarbazones. (b) Carry out the following conversions in not ...
... (i) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. (ii) There are two -NH2 groups in semicarbazide. However, only one is in volved in the formation of semicarbazones. (b) Carry out the following conversions in not ...
PIB and HH - Unit 4 - Chemical Names and Formulas
... Bonded atoms attain the stable electron configuration of a noble gas. The noble gases themselves exist as isolated atoms because that is their most stable condition. For the representative elements, the number of valence electrons is equal to the element’s group number in the periodic table. The tra ...
... Bonded atoms attain the stable electron configuration of a noble gas. The noble gases themselves exist as isolated atoms because that is their most stable condition. For the representative elements, the number of valence electrons is equal to the element’s group number in the periodic table. The tra ...
WRITING AP EQUATIONS AP equation sets are found in the free
... equation. All reactions do not fit neatly into the five types of reactions that you learned in Chemistry I. ...
... equation. All reactions do not fit neatly into the five types of reactions that you learned in Chemistry I. ...
Gas-phase reactivity of glycosides and methyl glycosides with Cu+
... animals and its redox chemistry is involved in a variety of oxidation processes. Copper deficiency may cause severe conditions such as anemia, coronary heart disease or bone fragility. Conversely, high levels of copper may also be toxic. Wilson’s disease,6 an inborn error of metabolism, causes toxic ...
... animals and its redox chemistry is involved in a variety of oxidation processes. Copper deficiency may cause severe conditions such as anemia, coronary heart disease or bone fragility. Conversely, high levels of copper may also be toxic. Wilson’s disease,6 an inborn error of metabolism, causes toxic ...
WRITING AP EQUATIONS AP equation sets are found in the
... equation. All reactions do not fit neatly into the five types of reactions that you learned in Chemistry I. ...
... equation. All reactions do not fit neatly into the five types of reactions that you learned in Chemistry I. ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.