Cyclam ``capa` POT.4` to ``capa` POT.3` denticity change
... with its metal center coordinated to the four nitrogens of the cyclam similar to the ruthenium nitrosyl tetraazamacrocyclic complexes trans-[Ru(NO)Cl(cyclam)]2+ 56 and trans-[Ru(NO)(OH)(tmc)](ClO4)2, (tmc ) 1,5,9,13-tetramethyl-1,5,9,13-tetraazacyclohexadecane).57 The complex investigated herein, th ...
... with its metal center coordinated to the four nitrogens of the cyclam similar to the ruthenium nitrosyl tetraazamacrocyclic complexes trans-[Ru(NO)Cl(cyclam)]2+ 56 and trans-[Ru(NO)(OH)(tmc)](ClO4)2, (tmc ) 1,5,9,13-tetramethyl-1,5,9,13-tetraazacyclohexadecane).57 The complex investigated herein, th ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... the greater component that keeps its state is called the solvent if both components start in the same state, the major component ...
... the greater component that keeps its state is called the solvent if both components start in the same state, the major component ...
CHAPTER 8 Chemical-Transport Models
... Primary PM is considered mostly chemically inert and is only affected by emission, transport and diffusion, and deposition processes; however, alkaline dust and sea salt can react with H2SO4 and HNO3. The processes leading to the formation of particulate SO4=, NO3- and NH4+ are known; they are gover ...
... Primary PM is considered mostly chemically inert and is only affected by emission, transport and diffusion, and deposition processes; however, alkaline dust and sea salt can react with H2SO4 and HNO3. The processes leading to the formation of particulate SO4=, NO3- and NH4+ are known; they are gover ...
enjoy chemistry
... larger amount of energy is required to remove electrons compared to Group 16 elements. (ii) Tendency to show –2 oxidation state diminishes from Sulphur to polonium in group 16. Ans:The outer electronic configuration of group 16 elements is ns2 np4. These elements therefore have the tendency to gain ...
... larger amount of energy is required to remove electrons compared to Group 16 elements. (ii) Tendency to show –2 oxidation state diminishes from Sulphur to polonium in group 16. Ans:The outer electronic configuration of group 16 elements is ns2 np4. These elements therefore have the tendency to gain ...
Low-Temperature Alkaline pH Hydrolysis of Oxygen-Free
... characterization of a composition of salts in the subsurface ocean and cryolava. From this new and original chemical composition, a laboratory study of several hydrolyses of tholins was carried out. The results obtained show the formation of many organic compounds, among them, species identified onl ...
... characterization of a composition of salts in the subsurface ocean and cryolava. From this new and original chemical composition, a laboratory study of several hydrolyses of tholins was carried out. The results obtained show the formation of many organic compounds, among them, species identified onl ...
The Mole Concept A. Atomic Masses and Avogadro`s Hypothesis 1
... Equal volumes of different gases, at the same temperature and pressure, contain the same number of particles. In other words, if 1 L of gas A reacts with 1 L of gas B, then there exactly the same number of particles of A and B present. Therefore, the molecule formed by reacting A with B is AB. Simil ...
... Equal volumes of different gases, at the same temperature and pressure, contain the same number of particles. In other words, if 1 L of gas A reacts with 1 L of gas B, then there exactly the same number of particles of A and B present. Therefore, the molecule formed by reacting A with B is AB. Simil ...
quantitative chemistry
... trying experiments out for yourself, you might be able to read in some books (if you had enough money to buy them - they were very expensive in those days) experiments other people had done, but there was probably little order to it. There was no theoretical paradigm underpinning observations, no fr ...
... trying experiments out for yourself, you might be able to read in some books (if you had enough money to buy them - they were very expensive in those days) experiments other people had done, but there was probably little order to it. There was no theoretical paradigm underpinning observations, no fr ...
Major 01 - KFUPM Faculty List
... molecules. Thus, assuming that 1 L N2 contains n molecules N2, then 2 L O2 must contain 2n O2 molecules and 1 L product must contain n product molecules: n N2 molecules and thus 2n N atoms + 2n O2 molecules and thus 4n O atoms n product molecules which thus must be n N2O4 molecules. ...
... molecules. Thus, assuming that 1 L N2 contains n molecules N2, then 2 L O2 must contain 2n O2 molecules and 1 L product must contain n product molecules: n N2 molecules and thus 2n N atoms + 2n O2 molecules and thus 4n O atoms n product molecules which thus must be n N2O4 molecules. ...
Chapter 3
... element the same on both sides of the equation. Do not change the subscripts. 3. Start by balancing those elements that appear in only one reactant and one product. 4. Balance those elements that appear in two or more reactants or products. 4. Remove all fractions (generally by multiplying everythin ...
... element the same on both sides of the equation. Do not change the subscripts. 3. Start by balancing those elements that appear in only one reactant and one product. 4. Balance those elements that appear in two or more reactants or products. 4. Remove all fractions (generally by multiplying everythin ...
File
... seen from the positive standard reduction potentials in Table 20.6, the halogens energetically favor the X form over the X2 form. Because the reduction potentials are so large, this give an indication of the relative ease to which halogens will grab electrons to form the halide ion. In general, the ...
... seen from the positive standard reduction potentials in Table 20.6, the halogens energetically favor the X form over the X2 form. Because the reduction potentials are so large, this give an indication of the relative ease to which halogens will grab electrons to form the halide ion. In general, the ...
Chemistry In action
... R. R. Donnelley/Jefferson City. The cover was printed by R. R. Donnelley/Jefferson City. The paper in this book was manufactured by a mill whose forest management programs include sustained yield—harvesting of its timberlands. Sustained yield harvesting principles ensure that the number of trees cut ...
... R. R. Donnelley/Jefferson City. The cover was printed by R. R. Donnelley/Jefferson City. The paper in this book was manufactured by a mill whose forest management programs include sustained yield—harvesting of its timberlands. Sustained yield harvesting principles ensure that the number of trees cut ...
Chapter 2 - hrsbstaff.ednet.ns.ca
... FA C T Magnesium plays a variety of roles in the body. It is involved in energy production, nerve function, and muscle relaxation, to name just a few. The magnesium in these tablets, like all naturally occurring magnesium, is made up of three isotopes. ...
... FA C T Magnesium plays a variety of roles in the body. It is involved in energy production, nerve function, and muscle relaxation, to name just a few. The magnesium in these tablets, like all naturally occurring magnesium, is made up of three isotopes. ...
Chapter 1 Introduction: Matter and Measurement
... Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early 19th century, championed by John Dalton. ...
... Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early 19th century, championed by John Dalton. ...
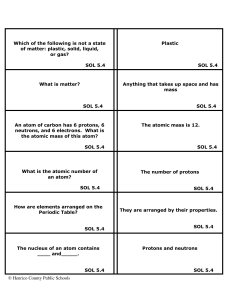
Matter Flashcards 5 - Henrico County Public Schools
... No, the properties of the compound are different from those of the elements that make it up. For instance, Sodium (Na) is an explosive substance and Chlorine (Cl) is a poisonous gas, but combined together, they make NaCl, or table salt, which is not poisonous or explosive. SOL 5.4 ...
... No, the properties of the compound are different from those of the elements that make it up. For instance, Sodium (Na) is an explosive substance and Chlorine (Cl) is a poisonous gas, but combined together, they make NaCl, or table salt, which is not poisonous or explosive. SOL 5.4 ...
Guide Kjeldahl
... History of the Kjeldahl Method For almost 130 years the determination of nitrogen by means of the method developed by the Danish chemist Johan Gustav Christoffer Thorsager Kjeldahl (1849–1900) has been an internationally accepted standard. The method was introduced in 1883 at a meeting of the Danish ...
... History of the Kjeldahl Method For almost 130 years the determination of nitrogen by means of the method developed by the Danish chemist Johan Gustav Christoffer Thorsager Kjeldahl (1849–1900) has been an internationally accepted standard. The method was introduced in 1883 at a meeting of the Danish ...
PART 3-ICHO 11-15
... individual compound of metal A obtained as a precipitate, was separated, thoroughly washed, dried and calcinated. The mass of the precipitate after the calcination to constant mass, was 0.3265 g. An aqueous ammonia solution was added in excess to the solution obtained after separation of the precipi ...
... individual compound of metal A obtained as a precipitate, was separated, thoroughly washed, dried and calcinated. The mass of the precipitate after the calcination to constant mass, was 0.3265 g. An aqueous ammonia solution was added in excess to the solution obtained after separation of the precipi ...
Document
... H2(g) collected. (Vapor pressure of water = 0.025 atm at 21C.) • (A) 0.283 g • (B) 435 g • (C) 0.571 g • (D) 7.14 g ...
... H2(g) collected. (Vapor pressure of water = 0.025 atm at 21C.) • (A) 0.283 g • (B) 435 g • (C) 0.571 g • (D) 7.14 g ...
PHOSPHORUS AND SULFUR COSMOCHEMISTRY
... the course of this research and writing of this dissertation. Virginia also assisted in edits of the document, preparation of a few figures, and provided much needed encouragement. I look forward to taking you to Hawai’i! The author also thanks Hansen’s Soda Company for their creation of the Monster ...
... the course of this research and writing of this dissertation. Virginia also assisted in edits of the document, preparation of a few figures, and provided much needed encouragement. I look forward to taking you to Hawai’i! The author also thanks Hansen’s Soda Company for their creation of the Monster ...
PREPARATION, STRUCTURAL STUDIES AND CHEMICAL
... widely used hypervalent iodine reagents for various chemical reactions.25 The IBX 5 and DMP 6 are employed as good oxidizing agents, especially in selective transformation of primary alcohols into aldehydes. However, they still have some drawbacks; IBX is insoluble in most useful organic solvents ex ...
... widely used hypervalent iodine reagents for various chemical reactions.25 The IBX 5 and DMP 6 are employed as good oxidizing agents, especially in selective transformation of primary alcohols into aldehydes. However, they still have some drawbacks; IBX is insoluble in most useful organic solvents ex ...
Chemical Vapor Deposition (CVD)
... • This technique is suitable for the manufacture of coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total p ...
... • This technique is suitable for the manufacture of coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total p ...
Sustainable Oxidation Catalysis for Synthesis
... Hydrogenability of a substrate, reaction routes determining selectivity, choice of metals and procedure of catalyst preparation, reaction conditions and changes of catalysts including deactivation are matters of discussion. A special focus is on the effect of water. Besides importance of a proper ma ...
... Hydrogenability of a substrate, reaction routes determining selectivity, choice of metals and procedure of catalyst preparation, reaction conditions and changes of catalysts including deactivation are matters of discussion. A special focus is on the effect of water. Besides importance of a proper ma ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.