Hydrogen Atoms under Magnification
... The development of quantum mechanics in the early part of the last century has had a profound influence on the way that scientists understand the world. Central to quantum mechanics is the concept of a wave function that satisfies the time-dependent Schrödinger equation [1]. According to the Copenh ...
... The development of quantum mechanics in the early part of the last century has had a profound influence on the way that scientists understand the world. Central to quantum mechanics is the concept of a wave function that satisfies the time-dependent Schrödinger equation [1]. According to the Copenh ...
CHAPTER 1: INTRODUCTION TO ENVIRONMENTAL CHEMISTRY
... Usually isotopes are referred to by their name (of symbol) and their mass number. Every element has at least 2 isotopes and some elements have as many as 25 isotopes. Example: The isotopes of hydrogen have separate names rather than being called hydrogen-1, hydrogen-2, etc. Their names are protium ( ...
... Usually isotopes are referred to by their name (of symbol) and their mass number. Every element has at least 2 isotopes and some elements have as many as 25 isotopes. Example: The isotopes of hydrogen have separate names rather than being called hydrogen-1, hydrogen-2, etc. Their names are protium ( ...
Hydrogen Atoms under Magnification: Direct
... The development of quantum mechanics in the early part of the last century has had a profound influence on the way that scientists understand the world. Central to quantum mechanics is the concept of a wave function that satisfies the time-dependent Schrödinger equation [1]. According to the Copenh ...
... The development of quantum mechanics in the early part of the last century has had a profound influence on the way that scientists understand the world. Central to quantum mechanics is the concept of a wave function that satisfies the time-dependent Schrödinger equation [1]. According to the Copenh ...
with answers
... Numerical answers that are given without showing any working or explanation will receive no marks. In general, short answers with keywords will be sufficient; long essays are not necessary! To illustrate or explain a point, a clear sketch is often sufficient! The maximum number of points for each qu ...
... Numerical answers that are given without showing any working or explanation will receive no marks. In general, short answers with keywords will be sufficient; long essays are not necessary! To illustrate or explain a point, a clear sketch is often sufficient! The maximum number of points for each qu ...
Document
... Mendeleev proposed the existence of an unknown element that he called eka-aluminum. This element is now called A. B. C. D. E. ...
... Mendeleev proposed the existence of an unknown element that he called eka-aluminum. This element is now called A. B. C. D. E. ...
Chapter 2.4 Periodic properties of the elements
... For calcium the first ionization energy (IE1), is 599 kJ/mol: Ca(g) + 599 kJ → Ca+(g) + eThe second ionization energy (IE2) is the amount of energy required to remove the second electron. For calcium, it may be represented as: Ca+(g) + 1145 kJ → Ca2+1(g) + eFor a given element, IE2 is always greater ...
... For calcium the first ionization energy (IE1), is 599 kJ/mol: Ca(g) + 599 kJ → Ca+(g) + eThe second ionization energy (IE2) is the amount of energy required to remove the second electron. For calcium, it may be represented as: Ca+(g) + 1145 kJ → Ca2+1(g) + eFor a given element, IE2 is always greater ...
6.1 The Waves Nature of Light
... 1. Electrons in an atom can only occupy certain orbits (corresponding to certain energies). 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. 3. Energy is only absorbed or emitted in such a way as to move an electron from one “allo ...
... 1. Electrons in an atom can only occupy certain orbits (corresponding to certain energies). 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. 3. Energy is only absorbed or emitted in such a way as to move an electron from one “allo ...
Example 4: A one-electron atom is irradiated with visible light. The
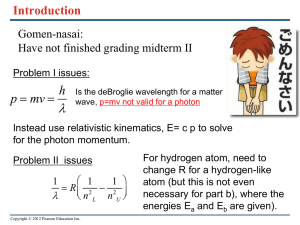
... Question 3.4: The emission spectrum of a one-electron atom showed 6 lines: 90000 cm-1, 75000 cm-1, 50000 cm-1, 40000 cm-1, 25000 cm-1 and 15000 cm-1. How many lines would you expect to see in the absorption spectrum and at what energies? Express your answer in cm-1. Question 2.5: An atom has three ...
... Question 3.4: The emission spectrum of a one-electron atom showed 6 lines: 90000 cm-1, 75000 cm-1, 50000 cm-1, 40000 cm-1, 25000 cm-1 and 15000 cm-1. How many lines would you expect to see in the absorption spectrum and at what energies? Express your answer in cm-1. Question 2.5: An atom has three ...
Lecture 2 - Tufts University
... prism, only a few lines are seen corresponding to discrete wavelengths. ...
... prism, only a few lines are seen corresponding to discrete wavelengths. ...
Chemistry
... o Be able to convert between units (dimensional analysis) o Be able to convert between the Celsius and Kelvin temperature scales o Understand and be able to solve density problems ...
... o Be able to convert between units (dimensional analysis) o Be able to convert between the Celsius and Kelvin temperature scales o Understand and be able to solve density problems ...
3-D Schrodinger`s Equation, Particle inside a 3
... To study how the x-ray spectra of atoms indicate the structure of these atoms ...
... To study how the x-ray spectra of atoms indicate the structure of these atoms ...
Review Questions for 1st year chemistry
... and 16), but the same number of protons (7). To find the neutrons, calculate mass ...
... and 16), but the same number of protons (7). To find the neutrons, calculate mass ...
Chapter 24. Organic Chemistry
... An ability of an atom to attract toward itself the electron cloud in a chemical bond Electronegativity is a relative concept, meaning that an electronegativilty of one atom can be measured relative to another atom Generally electronegativity increases from left to right acros a period in the periodi ...
... An ability of an atom to attract toward itself the electron cloud in a chemical bond Electronegativity is a relative concept, meaning that an electronegativilty of one atom can be measured relative to another atom Generally electronegativity increases from left to right acros a period in the periodi ...
Itty-Bitty Atoms
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
110 REVIEW MATERIALTro 2011
... Example 1: What is the specific gravity of ethanol? The density of ethanol = 0.791 g ethanol / 1 mL ethanol The density of water = 1.00 g H2O / 1 mL H2O ...
... Example 1: What is the specific gravity of ethanol? The density of ethanol = 0.791 g ethanol / 1 mL ethanol The density of water = 1.00 g H2O / 1 mL H2O ...
physical setting chemistry
... Base your answers to questions 76 through 78 on the information below. Carbon has three naturally occurring isotopes, C-12, C-13, and C-14. Diamond and graphite are familiar forms of solid carbon. Diamond is one of the hardest substances known, while graphite is a very soft substance. Diamond has a ...
... Base your answers to questions 76 through 78 on the information below. Carbon has three naturally occurring isotopes, C-12, C-13, and C-14. Diamond and graphite are familiar forms of solid carbon. Diamond is one of the hardest substances known, while graphite is a very soft substance. Diamond has a ...
Electronic Shells of Dirac Fermions in Graphene Quantum Rings in
... Figure 4 shows the calculated energy spectra for different total spin S for half-filled shell and effective dielectric constant κ = 6. We find that the ground state has total spin S = 1, separated by a very small gap from the total S = 0 state. In exactly 6-fold degenerate system we expect total gro ...
... Figure 4 shows the calculated energy spectra for different total spin S for half-filled shell and effective dielectric constant κ = 6. We find that the ground state has total spin S = 1, separated by a very small gap from the total S = 0 state. In exactly 6-fold degenerate system we expect total gro ...