Chemical Equilibrium
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
Continuous System Modeling - ETH
... The Computation of R and C I • The capacity of a long well insulated rod to conduct heat is proportional to the temperature gradient. · = ( · T) · S· = R · S· T = · Q· = · (T · S) ...
... The Computation of R and C I • The capacity of a long well insulated rod to conduct heat is proportional to the temperature gradient. · = ( · T) · S· = R · S· T = · Q· = · (T · S) ...
3.1 Thermal concepts (PPT)
... with the kinetic and potential energies of the molecules, i.e. how fast the molecules are vibrating and their chemical bonds. Heat goes from objects with high temperature to low temperature, not high thermal energy to low thermal energy. For example, a massive glacier will have more total thermal en ...
... with the kinetic and potential energies of the molecules, i.e. how fast the molecules are vibrating and their chemical bonds. Heat goes from objects with high temperature to low temperature, not high thermal energy to low thermal energy. For example, a massive glacier will have more total thermal en ...
Enthalpy
... reaction that forms 1 mol of the compound from its elements, with all substances in their standard states. The most stable form of the element is used. E.g. O2 not O3, C(graphite not diamond) The standard enthalpy of formation of the most stable form of any element is zero. Using Enthalpies of Forma ...
... reaction that forms 1 mol of the compound from its elements, with all substances in their standard states. The most stable form of the element is used. E.g. O2 not O3, C(graphite not diamond) The standard enthalpy of formation of the most stable form of any element is zero. Using Enthalpies of Forma ...
Chapter 6 - Chemistry
... - the energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings - as long as system and surroundings are in thermal contact, energy flows between them to establish temperature equality or thermal equilibrium - heat flows fr ...
... - the energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings - as long as system and surroundings are in thermal contact, energy flows between them to establish temperature equality or thermal equilibrium - heat flows fr ...
02.pure.substance
... To quantify the changes in the system, we have to be able to describe the substances which make up the system. The substance is characterized by its properties. This chapter shows how this is done for two major behavioral classes of substance covered in this course; phase-change fluids, and gases. ...
... To quantify the changes in the system, we have to be able to describe the substances which make up the system. The substance is characterized by its properties. This chapter shows how this is done for two major behavioral classes of substance covered in this course; phase-change fluids, and gases. ...
8.5 CONVECTION By convection we mean a motion of material due
... 1) If m2 cools to the same temperature as m1 the temperature gradient is adiabatic. 2) If m2 cools to a temperature above that of m1 the gradient is superadiabatic. 3) If m2 cools to a temperature below that of m1 , the gradient is subadiabatic What difference does this make? For the superadiabatic ...
... 1) If m2 cools to the same temperature as m1 the temperature gradient is adiabatic. 2) If m2 cools to a temperature above that of m1 the gradient is superadiabatic. 3) If m2 cools to a temperature below that of m1 , the gradient is subadiabatic What difference does this make? For the superadiabatic ...
8.5 CONVECTION By convection we mean a motion of material due
... 8.5 CONVECTION By convection we mean a motion of material due to buoyancy forces resulting from temperature differences. cold hot material is less dense than cold material. It rises hot The theory of convection is too complicated to get into here, but we may still understand the basic ideas. The fir ...
... 8.5 CONVECTION By convection we mean a motion of material due to buoyancy forces resulting from temperature differences. cold hot material is less dense than cold material. It rises hot The theory of convection is too complicated to get into here, but we may still understand the basic ideas. The fir ...
Energy
... Notice that water has a very high specific heat, whereas iron has a low specific heat. Water requires a lot of heat energy to raise its temperature. What is heat? Heat is thermal energy that flows from something at a higher temperature to something at a lower temperature. What is specific heat? ...
... Notice that water has a very high specific heat, whereas iron has a low specific heat. Water requires a lot of heat energy to raise its temperature. What is heat? Heat is thermal energy that flows from something at a higher temperature to something at a lower temperature. What is specific heat? ...
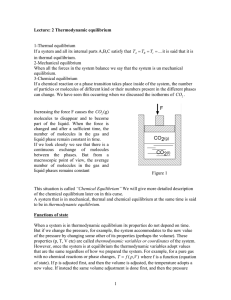
1 Lecture: 2 Thermodynamic equilibrium 1
... This situation is called “Chemical Equilibrium” We will give more detailed description of the chemical equilibrium later on in this curse. A system that is in mechanical, thermal and chemical equilibrium at the same time is said to be in thermodynamic equilibrium. Functions of state When a system is ...
... This situation is called “Chemical Equilibrium” We will give more detailed description of the chemical equilibrium later on in this curse. A system that is in mechanical, thermal and chemical equilibrium at the same time is said to be in thermodynamic equilibrium. Functions of state When a system is ...
Ppt19(PS8)_Thermo_Hess
... Assume the specific heat capacity of the dissolved NH4NO3 is negligible compared to water, an initial temperature of 25.0 C, and no heat transfer between the cold pack and the environment. dwater ~ 1.0 g/mL Q11 and Q12 on PS8b use similar ideas/skills. Try them now! Ppt19 ...
... Assume the specific heat capacity of the dissolved NH4NO3 is negligible compared to water, an initial temperature of 25.0 C, and no heat transfer between the cold pack and the environment. dwater ~ 1.0 g/mL Q11 and Q12 on PS8b use similar ideas/skills. Try them now! Ppt19 ...
The First, Second, and Third Law of Thermodynamics (ThLaws05.tex)
... The laws of thermodynamics apply to well-de…ned systems. First we will discuss a quite general form of the …rst and second law. I.e. we consider a system which is inhomogeneous, we allow mass transfer across the boundaries (open system), and we allow the boundaries to move. Fig.1 is a general repres ...
... The laws of thermodynamics apply to well-de…ned systems. First we will discuss a quite general form of the …rst and second law. I.e. we consider a system which is inhomogeneous, we allow mass transfer across the boundaries (open system), and we allow the boundaries to move. Fig.1 is a general repres ...
Slide 1
... a) temperature: 1-Autoclave: - steam heat to 121c at 1.5 atmospheric pressure for 15 min - autoclave treatment will inactivate all fungi, bacteria, viruses and also bacterial spores( quite resistant) ...
... a) temperature: 1-Autoclave: - steam heat to 121c at 1.5 atmospheric pressure for 15 min - autoclave treatment will inactivate all fungi, bacteria, viruses and also bacterial spores( quite resistant) ...
g - Valencia College
... cannot be created or destroyed. The energy of an isolated system must be ...
... cannot be created or destroyed. The energy of an isolated system must be ...
Heat transfer

Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.