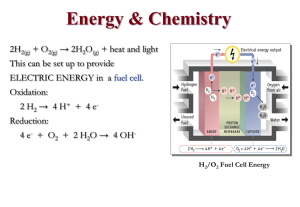
energy changes in physical and chemical processes
... internal energy within the substances, the molecules in the solid state always vibrate in their fixed positions. When the solid is heated, its temperature rises as the molecules absorb the heat energy. This heat energy absorbed is converted into vibrational energy and the molecules vibrate more vigo ...
... internal energy within the substances, the molecules in the solid state always vibrate in their fixed positions. When the solid is heated, its temperature rises as the molecules absorb the heat energy. This heat energy absorbed is converted into vibrational energy and the molecules vibrate more vigo ...
1 Problem T4 (Unified Thermodynamics): SOLUTIONS a) Describe
... provided energy (from the change in potential energy of the weight). The two chambers have received the energy, and it appears as increased internal energy of each of the chambers. b) What processes will you use to model this system? Why? (LO’s #2, #4, #5) The first process is not quasi-static but i ...
... provided energy (from the change in potential energy of the weight). The two chambers have received the energy, and it appears as increased internal energy of each of the chambers. b) What processes will you use to model this system? Why? (LO’s #2, #4, #5) The first process is not quasi-static but i ...
Chapter 06 - University of Windsor
... Force: "push" or "pull" exerted on an object. Work: energy required to overcome a force: Work = force x distance. Heat: ...
... Force: "push" or "pull" exerted on an object. Work: energy required to overcome a force: Work = force x distance. Heat: ...
Work Done by an Expanding Gas
... by the system. Suppose you push a large block with a certain force of magnitude over some distance. You have done work on the block; hence the energy of the block should increase. According to Newton's 3rd law, the block exerts the same magnitude of force , but in the opposite direction (i.e., direc ...
... by the system. Suppose you push a large block with a certain force of magnitude over some distance. You have done work on the block; hence the energy of the block should increase. According to Newton's 3rd law, the block exerts the same magnitude of force , but in the opposite direction (i.e., direc ...
What is Thermodynamics?
... from nano-scale molecular behaviour up to large scale planetary interactions, like environmental aspects. Its applications span a similarly large range of industrial domains (power generation, refrigeration, chemical reactions…); life sciences, with their complex molecular arrangements; nano–materia ...
... from nano-scale molecular behaviour up to large scale planetary interactions, like environmental aspects. Its applications span a similarly large range of industrial domains (power generation, refrigeration, chemical reactions…); life sciences, with their complex molecular arrangements; nano–materia ...
About the Guide - American Chemical Society
... Observing heat flow at the macroscopic level, then, we see that ideally the energy is conserved and that heat always flows from higher temperature to lower temperature. In other words, cooling down and warming up occur via the same mechanism—heat transfer from a higher temperature to a lower tempera ...
... Observing heat flow at the macroscopic level, then, we see that ideally the energy is conserved and that heat always flows from higher temperature to lower temperature. In other words, cooling down and warming up occur via the same mechanism—heat transfer from a higher temperature to a lower tempera ...
Epoxies and Glass Transition Temperature
... DMA is a procedure that is used to characterize the viscoelastic properties of materials. The main principle behind using DMA to define Tg is that the stiffness and damping (a measure of energy dissipation) of a polymeric material change significantly at the glass transition temperature. A controlle ...
... DMA is a procedure that is used to characterize the viscoelastic properties of materials. The main principle behind using DMA to define Tg is that the stiffness and damping (a measure of energy dissipation) of a polymeric material change significantly at the glass transition temperature. A controlle ...
Energy Notes
... • Thermodynamics is the study of energy. • The first law of thermodynamics states that the energy of the universe is constant. • Energy is neither created nor destroyed. • Energy can flow into (or out of) a system, but the amount gained (or lost) by the system is equal to the amount lost (or gained) ...
... • Thermodynamics is the study of energy. • The first law of thermodynamics states that the energy of the universe is constant. • Energy is neither created nor destroyed. • Energy can flow into (or out of) a system, but the amount gained (or lost) by the system is equal to the amount lost (or gained) ...
Thermal Stresses - Rick Bradford Home Page
... [5] Convective boundary condition: In this case we do not know in advance either the temperature or the heat flux at the boundary. Instead the temperature of a fluid in contact with the boundary is specified. The heat flux into the surface of the solid is then specified via a heat transfer coefficie ...
... [5] Convective boundary condition: In this case we do not know in advance either the temperature or the heat flux at the boundary. Instead the temperature of a fluid in contact with the boundary is specified. The heat flux into the surface of the solid is then specified via a heat transfer coefficie ...
chapter12
... Grades of Energy, cont. • If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B • When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy • Degradation of energy is the conversi ...
... Grades of Energy, cont. • If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B • When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy • Degradation of energy is the conversi ...
Heat transfer

Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.