AS Chemistry: Paper 2 Physical Inorganic Organic 1.0 Basic
... Produce reaction spider diagram for the halogenoalkanes: ...
... Produce reaction spider diagram for the halogenoalkanes: ...
Equilibrium - Clayton State University
... Assistant professor of chemistry Department of natural sciences Clayton state university ...
... Assistant professor of chemistry Department of natural sciences Clayton state university ...
fulltext $(function(){PrimeFaces.cw("Tooltip","widget_formSmash_items_resultList_20_j_idt799_0_j_idt801",{id:"formSmash:items:resultList:20:j_idt799:0:j_idt801",widgetVar:"widget_formSmash_items_resultList_20_j_idt799_0_j_idt801",showEffect:"fade",hideEffect:"fade",target:"formSmash:items:resultList:20:j_idt799:0:fullText"});});
... Fig. 1 Crystal structures of 3 and 4. The hydrogen atoms and BArF- are omitted; thermal ellipsoids are both shown at 50% probabilities. ...
... Fig. 1 Crystal structures of 3 and 4. The hydrogen atoms and BArF- are omitted; thermal ellipsoids are both shown at 50% probabilities. ...
File
... Part B: Reactions of Functional Groups (pg’s 65-80) 1a) Name some common groups of atoms and groups of atoms that can be added to a double or triple bond. b) List some of the possible classes of compounds that can be formed. 2) Explain using an example Markovnikov’s rule. Do alkynes follow this rule ...
... Part B: Reactions of Functional Groups (pg’s 65-80) 1a) Name some common groups of atoms and groups of atoms that can be added to a double or triple bond. b) List some of the possible classes of compounds that can be formed. 2) Explain using an example Markovnikov’s rule. Do alkynes follow this rule ...
Standard Voltages Cell Voltage
... • Standard half reaction voltages are determined by arbitrarily assigning the value of zero to the standard reduction half reaction for hydrogen ions to give hydrogen gas ...
... • Standard half reaction voltages are determined by arbitrarily assigning the value of zero to the standard reduction half reaction for hydrogen ions to give hydrogen gas ...
Thermodynamics and Equilibrium
... Figure 4.6 shows the effect that constraints (confinements or restrictions) on the degrees of freedom have on the density of states. The particles in Figure 4.6a are not constrained, so their energy is not constrained, which is shown by the complete shading. These particles are unbound and can have ...
... Figure 4.6 shows the effect that constraints (confinements or restrictions) on the degrees of freedom have on the density of states. The particles in Figure 4.6a are not constrained, so their energy is not constrained, which is shown by the complete shading. These particles are unbound and can have ...
Document
... the energy released when bonds form. ie. energy is absorbed exothermic reaction - the energy required to break bonds is less than the energy released when bonds form. ie. energy is produced ...
... the energy released when bonds form. ie. energy is absorbed exothermic reaction - the energy required to break bonds is less than the energy released when bonds form. ie. energy is produced ...
Chapter 11 - Alcohols and Ethers1
... alcohols and methanol react to form alkyl halides in concentrated ...
... alcohols and methanol react to form alkyl halides in concentrated ...
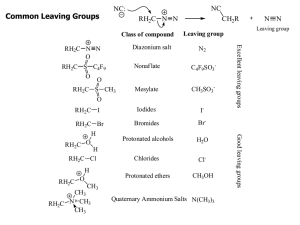
Common Leaving Groups
... •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It i ...
... •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It i ...
INDIAN JOURNAL OF CHEMISTRY
... complexes are H+ dependent. It is suggested that the complexes are formed by hydrogen bonds, P-H …. O-Mn, and the redox involve the dissociation of H from the PH bond causing transfer of two electrons from substrate to MnO4 through O bridge. The relative stability of the complex C2 > C1 because ...
... complexes are H+ dependent. It is suggested that the complexes are formed by hydrogen bonds, P-H …. O-Mn, and the redox involve the dissociation of H from the PH bond causing transfer of two electrons from substrate to MnO4 through O bridge. The relative stability of the complex C2 > C1 because ...
10 PRE-LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is t
... Thermochemistry is the study of the relationship between chemical reactions and energy changes. Thermochemistry has many practical applications. For example, using thermochemistry: (1) mining engineers can calculate how much fuel will be needed to prepare metals from their ores, (2) structural ...
... Thermochemistry is the study of the relationship between chemical reactions and energy changes. Thermochemistry has many practical applications. For example, using thermochemistry: (1) mining engineers can calculate how much fuel will be needed to prepare metals from their ores, (2) structural ...