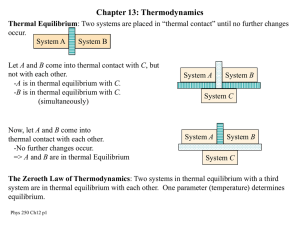
Heat and Thermodynamics
... Conduction is heat transfer by means of molecular agitation within a material without any motion of the material as a whole. If one end of a metal rod is at a higher temperature, then energy will be transferred down the rod toward the colder end because the higher speed particles will collide with t ...
... Conduction is heat transfer by means of molecular agitation within a material without any motion of the material as a whole. If one end of a metal rod is at a higher temperature, then energy will be transferred down the rod toward the colder end because the higher speed particles will collide with t ...
Thermodynamics - Clayton State University
... Any form of energy is transformable into any other form. This includes heat energy. However, it is much easier to convert useful mechanical energy into heat energy than it is to convert heat energy into useful mechanical energy. Heat energy is based on random movement of molecules, so it is a disorg ...
... Any form of energy is transformable into any other form. This includes heat energy. However, it is much easier to convert useful mechanical energy into heat energy than it is to convert heat energy into useful mechanical energy. Heat energy is based on random movement of molecules, so it is a disorg ...
Thermodynamics Energy Changes
... endothermic and the energy term would be written on the left side of the equation. ...
... endothermic and the energy term would be written on the left side of the equation. ...
Chapter 19 – The First Law of Thermodynamics
... Interesting Note: If a system changes from an initial state i to a final state f along different paths (e.g., Path A and Path B), the change in internal energy will be the same along those paths. And, in fact, all paths that go from i to f. That is, U = Uf - Ui . From the first law, that means that ...
... Interesting Note: If a system changes from an initial state i to a final state f along different paths (e.g., Path A and Path B), the change in internal energy will be the same along those paths. And, in fact, all paths that go from i to f. That is, U = Uf - Ui . From the first law, that means that ...
Radiation
... air sinks. The uneven distribution of heat creates more air currents. Remember: Heat is all around you; it's transferred in three ways. ...
... air sinks. The uneven distribution of heat creates more air currents. Remember: Heat is all around you; it's transferred in three ways. ...
Ch 16 Thermal Energy and Heat
... • In the 1700’s scientists thought heat was a fluid called a caloric that flowed between objects. • In 1798, the scientist Count Rumford concluded, from his observations, that heat could not be a kind of matter but instead was related to the motion of objects ...
... • In the 1700’s scientists thought heat was a fluid called a caloric that flowed between objects. • In 1798, the scientist Count Rumford concluded, from his observations, that heat could not be a kind of matter but instead was related to the motion of objects ...
Process
... The total energy of a system is the Internal Energy, U. The internal energy is a state function. If a system as an initial energy Ui and after a transformation as a n energy Uf then the variation of internal energy, U is: ...
... The total energy of a system is the Internal Energy, U. The internal energy is a state function. If a system as an initial energy Ui and after a transformation as a n energy Uf then the variation of internal energy, U is: ...
Second Law of thermodynamics
... enters or leaves (but energy may be exchanged with the environment) Ex: idealized systems studied in physics • Open system is one for which mass may enter or leave (as well as energy) Ex: plants and animals ...
... enters or leaves (but energy may be exchanged with the environment) Ex: idealized systems studied in physics • Open system is one for which mass may enter or leave (as well as energy) Ex: plants and animals ...
PHYS 1220, Engineering Physics, Chapter 19 – The First Law of
... • A status is to describe the states of a system. It does not depend on the history of the system, rather it only depends on its thermodynamic state (e.g. temperature, volume, pressure, number of molecules etc.) - What is a “process”? • A process is to describe how the system evolve from one state ( ...
... • A status is to describe the states of a system. It does not depend on the history of the system, rather it only depends on its thermodynamic state (e.g. temperature, volume, pressure, number of molecules etc.) - What is a “process”? • A process is to describe how the system evolve from one state ( ...
HeatTransfer
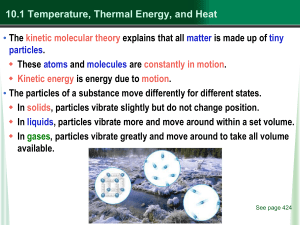
... These atoms and molecules are constantly in motion. Kinetic energy is energy due to motion. • The particles of a substance move differently for different states. In solids, particles vibrate slightly but do not change position. In liquids, particles vibrate more and move around within a set ...
... These atoms and molecules are constantly in motion. Kinetic energy is energy due to motion. • The particles of a substance move differently for different states. In solids, particles vibrate slightly but do not change position. In liquids, particles vibrate more and move around within a set ...
PY2104 - Introduction to thermodynamics and Statistical physics
... where C is constant (known as Curie constant). Its internal energy is U = −M B . The increment of work done by the system upon the external environment is dW = M dB. (i) Write an expression for the heat input dQ to the system in terms of thermodynamic variables, M and B (more precisely, dM and dB). ...
... where C is constant (known as Curie constant). Its internal energy is U = −M B . The increment of work done by the system upon the external environment is dW = M dB. (i) Write an expression for the heat input dQ to the system in terms of thermodynamic variables, M and B (more precisely, dM and dB). ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.