Enthalpy of Neutralization
... Experiment #12. Enthalpy of Neutralization Introduction In the course of most physical processes and chemical reactions there is a change in energy. In chemistry what is normally measured is H (enthalpy change), the change in heat at constant pressure and ignoring any work done by the reacting syst ...
... Experiment #12. Enthalpy of Neutralization Introduction In the course of most physical processes and chemical reactions there is a change in energy. In chemistry what is normally measured is H (enthalpy change), the change in heat at constant pressure and ignoring any work done by the reacting syst ...
Document
... more work is done by the system than heat was flowing into the system. 2) The internal energy of a system decreases when work is done on the system and heat is flowing into the system. 3) The system does work on the surroundings when an ideal gas expands against a constant external pressure. 4) All ...
... more work is done by the system than heat was flowing into the system. 2) The internal energy of a system decreases when work is done on the system and heat is flowing into the system. 3) The system does work on the surroundings when an ideal gas expands against a constant external pressure. 4) All ...
gec221 tutorial kit - Covenant University
... gas law and calculate the compressibility factor by using the ideal gas law. Also calculate the volume by using virial equation of state truncated at second term and compressibility factor Z by using virial equation of state truncated at second term. A substance expands from V1 = 1ft3 to V2 = 6 ft3 ...
... gas law and calculate the compressibility factor by using the ideal gas law. Also calculate the volume by using virial equation of state truncated at second term and compressibility factor Z by using virial equation of state truncated at second term. A substance expands from V1 = 1ft3 to V2 = 6 ft3 ...
heat engine
... Refrigerators, air conditioners, and heat pumps are devices that make heat flow from cold to hot. This is called the refrigeration process. ...
... Refrigerators, air conditioners, and heat pumps are devices that make heat flow from cold to hot. This is called the refrigeration process. ...
Document
... U (or any state function) can be expressed as an infinitesimal quantity, dU, that when integrated, depends only on the initial and final states. dU is called an exact differential. ...
... U (or any state function) can be expressed as an infinitesimal quantity, dU, that when integrated, depends only on the initial and final states. dU is called an exact differential. ...
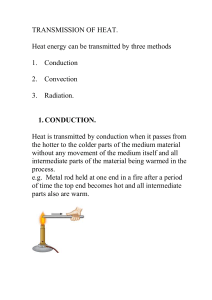
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.