Interpreting Graphs
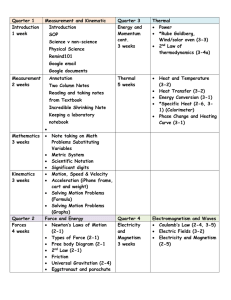
... Solving motion problems graphically Calculate average speed graphically as slope on a distance graph between two points. Use the computer as a lab instrument to take data and aid in the analysis of motion via graphing Calculate average speed and velocity algebraically and graphically. Calcul ...
... Solving motion problems graphically Calculate average speed graphically as slope on a distance graph between two points. Use the computer as a lab instrument to take data and aid in the analysis of motion via graphing Calculate average speed and velocity algebraically and graphically. Calcul ...
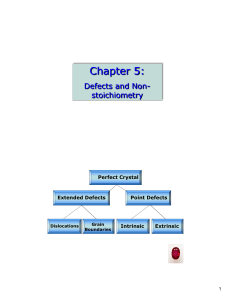
15-1 Note 15 Properties of Bulk Matter
... described in terms of a grouping of atoms called a molecule. To form a molecule two or more atoms bind together via a combination of electrostatic and quantum mechanical processes that we shall not go into here. The complexity of molecules varies widely from the simplest, hydrogen gas, which consist ...
... described in terms of a grouping of atoms called a molecule. To form a molecule two or more atoms bind together via a combination of electrostatic and quantum mechanical processes that we shall not go into here. The complexity of molecules varies widely from the simplest, hydrogen gas, which consist ...
Screen Version
... Carnot’s cycle consists of taking the working substance in the cylinder through the following four operations that together constitute a reversible, cyclic transformation 1. The substance starts at point A with temperature T2. The working substance is compressed adiabatically to state B. Its temper ...
... Carnot’s cycle consists of taking the working substance in the cylinder through the following four operations that together constitute a reversible, cyclic transformation 1. The substance starts at point A with temperature T2. The working substance is compressed adiabatically to state B. Its temper ...
a level physics paper one
... A load of 60N is applied to a steel wire of length 2.5m and cross-sectional area of 0.22mm2. If Young’s Modulus for steel is 210GPa, find the expansion ...
... A load of 60N is applied to a steel wire of length 2.5m and cross-sectional area of 0.22mm2. If Young’s Modulus for steel is 210GPa, find the expansion ...
constraints on heat production and thickness of continental plates
... plausible model, the relationships between temperature, heat production and plate thickness, including some absolute limits on heat production. The relationships can be used to evaluate the implications of various independent estimates of these quantities, and some examples are discussed here. An ob ...
... plausible model, the relationships between temperature, heat production and plate thickness, including some absolute limits on heat production. The relationships can be used to evaluate the implications of various independent estimates of these quantities, and some examples are discussed here. An ob ...
2. Prediction of Crystal Structures
... diffusion-equation [8] and cluster methods [9]. Frequently combinations of these methods are used [10,12]. Since the energy hypersurface has a large number of local minima, the 'classical' minimization methods like steepest descent require several hundred runs starting from different points. These s ...
... diffusion-equation [8] and cluster methods [9]. Frequently combinations of these methods are used [10,12]. Since the energy hypersurface has a large number of local minima, the 'classical' minimization methods like steepest descent require several hundred runs starting from different points. These s ...
Chapter Two The Thermodynamic Laws
... reservoir and produce a net amount of work." This was shown to be equivalent to the statement of Clausius. (2.3.2). Statements of the second law (2.3.2.1). Thermal reservoir Thermal reservoir, characterized by its temperature, is a reservoir of infinite heat capacity. Thermal reservoir can play the ...
... reservoir and produce a net amount of work." This was shown to be equivalent to the statement of Clausius. (2.3.2). Statements of the second law (2.3.2.1). Thermal reservoir Thermal reservoir, characterized by its temperature, is a reservoir of infinite heat capacity. Thermal reservoir can play the ...
Chapter 8 Problems - University of Colorado Colorado Springs
... A daredevil plans to bungee-jump from a balloon 65.0 m above a carnival midway. He will use a uniform elastic cord, tied to a harness around his body, to stop his fall at a point 10.0 m above the ground. Model his body as a particle, and the cord as having negligible mass and one that obeys Hooke’s ...
... A daredevil plans to bungee-jump from a balloon 65.0 m above a carnival midway. He will use a uniform elastic cord, tied to a harness around his body, to stop his fall at a point 10.0 m above the ground. Model his body as a particle, and the cord as having negligible mass and one that obeys Hooke’s ...
Review of Thermodynamics
... method. Whereas it is not possible to predict the equation of state without knowing the microscopic structure of a system, it is nevertheless possible to predict many apparently unrelated macroscopic quantities and the relationships between them given the fundamental relation between its state varia ...
... method. Whereas it is not possible to predict the equation of state without knowing the microscopic structure of a system, it is nevertheless possible to predict many apparently unrelated macroscopic quantities and the relationships between them given the fundamental relation between its state varia ...
10 Simple Harmonic Motion
... We can find the spring constant k for this spring by taking the ratio of the force to the stretch for a particular interval. In other words, we can find the slope of the F vs. x graph for each spring. The slope of the line and the spring constant for spring is 50 N/m. As on any other F vs. x graph, ...
... We can find the spring constant k for this spring by taking the ratio of the force to the stretch for a particular interval. In other words, we can find the slope of the F vs. x graph for each spring. The slope of the line and the spring constant for spring is 50 N/m. As on any other F vs. x graph, ...
Chapter 5 Thermochemistry
... calorimeter, the heat change for the system can be found by measuring the heat change for the water in the calorimeter. • The specific heat for water is well known (4.184 J/g∙K). • We can calculate H for the reaction with this equation: q = m Cs T Thermochemistry © 2015 Pearson Education ...
... calorimeter, the heat change for the system can be found by measuring the heat change for the water in the calorimeter. • The specific heat for water is well known (4.184 J/g∙K). • We can calculate H for the reaction with this equation: q = m Cs T Thermochemistry © 2015 Pearson Education ...
III.3 Momentum balance: Euler and Navier–Stokes equations
... constant volume (CV ). Since V is proportional to 1/ρ, this so-called “adiabatic equation of state” provides the needed constraint relating pressure and mass density. Eventually, one may argue that non-relativistic physics automatically implies a further conservation law besides those for mass and l ...
... constant volume (CV ). Since V is proportional to 1/ρ, this so-called “adiabatic equation of state” provides the needed constraint relating pressure and mass density. Eventually, one may argue that non-relativistic physics automatically implies a further conservation law besides those for mass and l ...
Work
... In non-isolated systems, energy crosses the boundary of the system during some time interval due to an interaction with the environment. Work – transfers energy by applying a force and causing a displacement of the point of application of the force. Mechanical Wave – transfers energy by allowing a d ...
... In non-isolated systems, energy crosses the boundary of the system during some time interval due to an interaction with the environment. Work – transfers energy by applying a force and causing a displacement of the point of application of the force. Mechanical Wave – transfers energy by allowing a d ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.