Metal-Ligand and Metal-Metal Bonding Core Module 4 RED
... understand the relationship between CO, the 'classic' π-acceptor and related ligands such as NO, CN, and N2. describe the Dewar-Duncanson –Chatt model for metal-alkene and metal-alkyne bonding. understand the affect of metal binding on the reactivity of a coordinated alkene. describe the nature of t ...
... understand the relationship between CO, the 'classic' π-acceptor and related ligands such as NO, CN, and N2. describe the Dewar-Duncanson –Chatt model for metal-alkene and metal-alkyne bonding. understand the affect of metal binding on the reactivity of a coordinated alkene. describe the nature of t ...
The Final Secret of Free Energy
... with a localized set of mass, we shall be concerned with the local energy density (joules/coulomb) of the potential. But forget the conventional myth of visualizing the potential as pushing a unit charge in from infinity "against the force field" -- there isn't any force field in the vacuum, as is w ...
... with a localized set of mass, we shall be concerned with the local energy density (joules/coulomb) of the potential. But forget the conventional myth of visualizing the potential as pushing a unit charge in from infinity "against the force field" -- there isn't any force field in the vacuum, as is w ...
Work Done On or By a Gas
... issues associated with internal combustion engines are important issues. A partial solution to these problems would be more efficient engines. • What does the term efficiency mean when applied to engines? • How efficient do you think present engines are when given as a percentage? • Can the efficien ...
... issues associated with internal combustion engines are important issues. A partial solution to these problems would be more efficient engines. • What does the term efficiency mean when applied to engines? • How efficient do you think present engines are when given as a percentage? • Can the efficien ...
as PDF
... (Z=57) in its neutral state, becomes La3+ with the electron configuration of Xe (Z=54): ls22s22p63s23p64s23d104p65s24dl05p6. In words, this electron configuration may be described as: “two electrons are in the first shell occupying the simplest type of orbital (1s2); eight in the second shell, two o ...
... (Z=57) in its neutral state, becomes La3+ with the electron configuration of Xe (Z=54): ls22s22p63s23p64s23d104p65s24dl05p6. In words, this electron configuration may be described as: “two electrons are in the first shell occupying the simplest type of orbital (1s2); eight in the second shell, two o ...
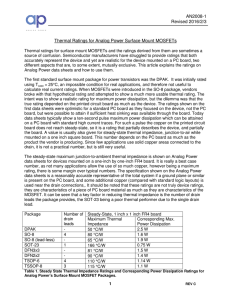
Thermal Ratings of Surface Mount Packages
... Since these thermal impedance numbers are PC board dependent, they are affected by the amount of copper close to the device. The accuracy depends on just how much copper is present. Smaller packages tend to be more limited in this respect, but that is, to some extent, included in the rating. For a p ...
... Since these thermal impedance numbers are PC board dependent, they are affected by the amount of copper close to the device. The accuracy depends on just how much copper is present. Smaller packages tend to be more limited in this respect, but that is, to some extent, included in the rating. For a p ...
(s) + H 2 (g) - Gordon State College
... • DSsys for a spontaneous process can be less than 0 as long as DSsurr > 0. This would make DSuniv still (+). • For an isolated system, DSsys = 0 for a reversible process and DSsys > 0 for a spontaneous process. Prentice Hall © 2003 ...
... • DSsys for a spontaneous process can be less than 0 as long as DSsurr > 0. This would make DSuniv still (+). • For an isolated system, DSsys = 0 for a reversible process and DSsys > 0 for a spontaneous process. Prentice Hall © 2003 ...
Kinetics and Equilibrium ___ 1. In a chemical reaction the use of a
... of the equilibrium constant; (2) increasing the energy of the products; (3) decreasing the energy of the products; (4) decreasing the required activation energy. ___ 42. For a chemical system at equilibrium a rise in temperature will (1) favor the endothermic reaction; (2) favor the exothermic react ...
... of the equilibrium constant; (2) increasing the energy of the products; (3) decreasing the energy of the products; (4) decreasing the required activation energy. ___ 42. For a chemical system at equilibrium a rise in temperature will (1) favor the endothermic reaction; (2) favor the exothermic react ...
Chemistry 180-213B Introductory Physical
... We suppose, to correspond to the actual state of affairs, that the pressure of the air in the room always equals that of the external air. In the usual notation, the (inner, thermal) energy is, per unit mass,* u = cvT . ...
... We suppose, to correspond to the actual state of affairs, that the pressure of the air in the room always equals that of the external air. In the usual notation, the (inner, thermal) energy is, per unit mass,* u = cvT . ...
Erbium Spectroscopy and 1.5- m Emission in KGd(WO4 )2: Er,Yb
... ANY researchers into laser systems focus on lasers operating in the infrared spectral range, especially around 1.5 and 3 m (6667 and 3333 cm ) for use in, for example, optical communications, medicine, and light detection and ranging (LIDAR) [1], [2]. Of these, solid-state lasers are preferred for m ...
... ANY researchers into laser systems focus on lasers operating in the infrared spectral range, especially around 1.5 and 3 m (6667 and 3333 cm ) for use in, for example, optical communications, medicine, and light detection and ranging (LIDAR) [1], [2]. Of these, solid-state lasers are preferred for m ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.