Equilibrium Statistical Mechanics
... Energy Maximum Entropy Maximum Work and Heat Engines Thermodynamic potentials Specific heats Gibbs-Duhem Stability conditions ...
... Energy Maximum Entropy Maximum Work and Heat Engines Thermodynamic potentials Specific heats Gibbs-Duhem Stability conditions ...
3.8 Useful Relationships - Molecular Diversity Preservation
... The microscopic forms of energy are those related to the molecular structure of a system and the degree of the molecular activity, and they are independent of outside reference frames. The sum of all the microscopic forms of energy is called the internal energy of a system. The internal energy of ...
... The microscopic forms of energy are those related to the molecular structure of a system and the degree of the molecular activity, and they are independent of outside reference frames. The sum of all the microscopic forms of energy is called the internal energy of a system. The internal energy of ...
Energy, Entropy and Exergy Concepts and Their Roles in Thermal
... • The microscopic forms of energy are those related to the molecular structure of a system and the degree of the molecular activity, and they are independent of outside reference frames. The sum of all the microscopic forms of energy is called the internal energy of a system. The internal energy of ...
... • The microscopic forms of energy are those related to the molecular structure of a system and the degree of the molecular activity, and they are independent of outside reference frames. The sum of all the microscopic forms of energy is called the internal energy of a system. The internal energy of ...
Symmetry axis n
... It is also called rotational axis, if a rotation around an axis by 360°/n results in a molecule indistinguishable from the original. Examples are water (C2 ) and ammonia (C3 ). A molecule can have more than one symmetry axis and the one with the highest number of n is called the principal axis and t ...
... It is also called rotational axis, if a rotation around an axis by 360°/n results in a molecule indistinguishable from the original. Examples are water (C2 ) and ammonia (C3 ). A molecule can have more than one symmetry axis and the one with the highest number of n is called the principal axis and t ...
Mechanism of ultra low friction of multilayer graphene
... The question is then the mechanism of friction between the transfer film and the graphite surface. Does the friction occur inside the transfer film similar to the macroscopic friction of the pile of papers? If not, what is the difference in the friction mechanism between general solid materials and ...
... The question is then the mechanism of friction between the transfer film and the graphite surface. Does the friction occur inside the transfer film similar to the macroscopic friction of the pile of papers? If not, what is the difference in the friction mechanism between general solid materials and ...
Interrelation of work function and surface stability: the case of BaAl4
... for the Fermi level (EF ) to be accurate. A more efficient method is to link the surface calculation to a bulk one where EF is very accurate. The average potential in the bulk (< V >bulk ) is set equal to the average potential in the middle of the slab. Accuracies of a few hundredths of an eV can th ...
... for the Fermi level (EF ) to be accurate. A more efficient method is to link the surface calculation to a bulk one where EF is very accurate. The average potential in the bulk (< V >bulk ) is set equal to the average potential in the middle of the slab. Accuracies of a few hundredths of an eV can th ...
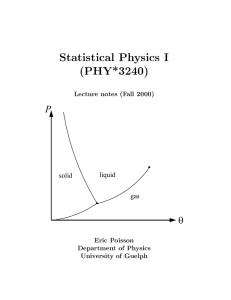
book - University of Guelph Physics
... and theoretically, because their physical properties do not change with time. The framework of thermodynamics applies equally well to all such macroscopic systems; it is a powerful and very general framework. An example of a thermodynamic system is a fluid (a gas or a liquid) confined to a beaker of ...
... and theoretically, because their physical properties do not change with time. The framework of thermodynamics applies equally well to all such macroscopic systems; it is a powerful and very general framework. An example of a thermodynamic system is a fluid (a gas or a liquid) confined to a beaker of ...
UNIVERSITY OF CALICUT (Abstract)
... another supervisor has to be appointed.However the existing work load should be maintained. Guidelines for doing project The project work provides the opportunity to study a topic in depth that has been chosen or which has been suggested by a staff member.The students first carryout a literature sur ...
... another supervisor has to be appointed.However the existing work load should be maintained. Guidelines for doing project The project work provides the opportunity to study a topic in depth that has been chosen or which has been suggested by a staff member.The students first carryout a literature sur ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.