OpenStax Physics Text for 2B - Chapter 10
... Now consider the effects of eating. Eating increases the internal energy of the body by adding chemical potential energy (this is an unromantic view of a good steak). The body metabolizes all the food we consume. Basically, metabolism is an oxidation process in which the chemical potential energy of ...
... Now consider the effects of eating. Eating increases the internal energy of the body by adding chemical potential energy (this is an unromantic view of a good steak). The body metabolizes all the food we consume. Basically, metabolism is an oxidation process in which the chemical potential energy of ...
transition state
... • propagation steps: reactive intermediates react with stable molecules to generate other reactive intermediates (allows chain to continue) • termination step: side-reactions that slow the reaction; usually combination of two reactive intermediates into one stable molecule ...
... • propagation steps: reactive intermediates react with stable molecules to generate other reactive intermediates (allows chain to continue) • termination step: side-reactions that slow the reaction; usually combination of two reactive intermediates into one stable molecule ...
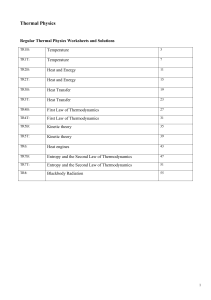
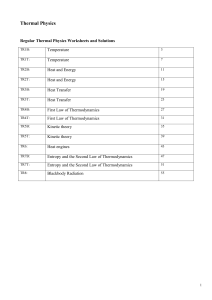
Temperature
... Any macroscopic body composed of a large number of particles has internal energy U associated with internal degrees of freedom . (e.g kinetic energy of molecules in a volume of gas, vibrational energy of atoms in a crystal. The internal energy U(T) is in general a complicated function of T and is di ...
... Any macroscopic body composed of a large number of particles has internal energy U associated with internal degrees of freedom . (e.g kinetic energy of molecules in a volume of gas, vibrational energy of atoms in a crystal. The internal energy U(T) is in general a complicated function of T and is di ...
Thermodynamic Considerations in Animal Nutrition Department of
... Department of Zoology, University of Georgia, Athens, Georgia 30601 SYNOPSIS. Acquisition of energy is a prime objective of the search for nutrition. The energy budget of a population or trophic level comprises the sum of energy gains and losses by each individual organism. Since these energy exchan ...
... Department of Zoology, University of Georgia, Athens, Georgia 30601 SYNOPSIS. Acquisition of energy is a prime objective of the search for nutrition. The energy budget of a population or trophic level comprises the sum of energy gains and losses by each individual organism. Since these energy exchan ...
Pg 65 The student`s spreadsheet is shown in Fig. 12.5. A B C D 1
... 13. Smoke particles in a transparent box are observed using a microscope. A small point of light is seen to move around as shown. -insert diagramWhat does this experiment demonstrate about air molecules? A B C D ...
... 13. Smoke particles in a transparent box are observed using a microscope. A small point of light is seen to move around as shown. -insert diagramWhat does this experiment demonstrate about air molecules? A B C D ...
Temperature
... Hold the beaker in your hands. Explain what you observe. 4. Thermal Expansion of solids - bimetallic strip Heat the strip using the hairdryer or hot air gun. What happens to the strip, and why? Can you think of a use for such a strip? C. Quantitative Questions: 1. Aluminium rivets used in aeroplane ...
... Hold the beaker in your hands. Explain what you observe. 4. Thermal Expansion of solids - bimetallic strip Heat the strip using the hairdryer or hot air gun. What happens to the strip, and why? Can you think of a use for such a strip? C. Quantitative Questions: 1. Aluminium rivets used in aeroplane ...
Atomic Structure
... Hold the beaker in your hands. Explain what you observe. 4. Thermal Expansion of solids - bimetallic strip Heat the strip using the hairdryer or hot air gun. What happens to the strip, and why? Can you think of a use for such a strip? C. Quantitative Questions: 1. Aluminium rivets used in aeroplane ...
... Hold the beaker in your hands. Explain what you observe. 4. Thermal Expansion of solids - bimetallic strip Heat the strip using the hairdryer or hot air gun. What happens to the strip, and why? Can you think of a use for such a strip? C. Quantitative Questions: 1. Aluminium rivets used in aeroplane ...
試料作製・評価による希土類化合物の研究 The research of sample preparation and measurement of
... Sommerfeld coefficient γ = 200 mJ/(K2 mole) of Yb4As3 does not originate from impurities. The change of γ is observed when the specific heat is measured in a magnetic field of 1 T. The Korringa law (T1T=constant) holds only in the narrow temperature range between 20 K and 10 K. Their physical proper ...
... Sommerfeld coefficient γ = 200 mJ/(K2 mole) of Yb4As3 does not originate from impurities. The change of γ is observed when the specific heat is measured in a magnetic field of 1 T. The Korringa law (T1T=constant) holds only in the narrow temperature range between 20 K and 10 K. Their physical proper ...
Finite Temperature Field Theory
... Any condensed matter system is confined to the compact domain MD of the space RD , with the boundary B D−1 . The effects of boundary and edges of a spatial domain should always be accounted for. Experiments show the size and boundary effects of a condensed matter system can be large and even leading ...
... Any condensed matter system is confined to the compact domain MD of the space RD , with the boundary B D−1 . The effects of boundary and edges of a spatial domain should always be accounted for. Experiments show the size and boundary effects of a condensed matter system can be large and even leading ...
GHW#12-Chapter-6-Tro
... – To a biologist: • Energy is responsible for growth and development of an organism. – To a chemist: • Energy is the ability to do work or transfer heat. ...
... – To a biologist: • Energy is responsible for growth and development of an organism. – To a chemist: • Energy is the ability to do work or transfer heat. ...
PHYS P1 - free kcse past papers
... maintained at 3.0 X 106Nm-2, calculate the force with which the water jets out of the hole.(3 marks) ...
... maintained at 3.0 X 106Nm-2, calculate the force with which the water jets out of the hole.(3 marks) ...
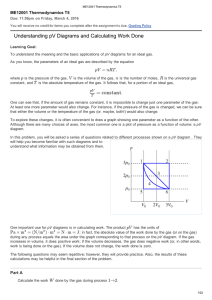
Document
... The macroscopic energy of a system is associated with the motion of the system as a whole and defined as Emech = K+U (this is the energy you were taught in Phys 4A). The microscopic energy within the system is called the internal energy, that’s the energy inside the object (or thermal energy) wh ...
... The macroscopic energy of a system is associated with the motion of the system as a whole and defined as Emech = K+U (this is the energy you were taught in Phys 4A). The microscopic energy within the system is called the internal energy, that’s the energy inside the object (or thermal energy) wh ...
Final Exam Review Questions PHY 2425
... Which of the following variables are state variables? A) P, V, and T D) (A) and (B) B) Internal energy, U E) (A), (B), and (C) C) W and Q Ans: D Section: 18–3 Topic: Joule's Experiment and the First Law... Type: Conceptual 54 The first law of thermodynamics has as its basis the same fundamental prin ...
... Which of the following variables are state variables? A) P, V, and T D) (A) and (B) B) Internal energy, U E) (A), (B), and (C) C) W and Q Ans: D Section: 18–3 Topic: Joule's Experiment and the First Law... Type: Conceptual 54 The first law of thermodynamics has as its basis the same fundamental prin ...
The Fluctuation-imposed Limit for Temperature Measurement
... is to be measured, is assumed to be isothermal and to have infinite heat capacity. The thermometer incorporates an easily measured quantity, like the magnetization of a salt, whose mean value is a function of temperature and can thus be used to infer the reservoir temperature. From statistical mecha ...
... is to be measured, is assumed to be isothermal and to have infinite heat capacity. The thermometer incorporates an easily measured quantity, like the magnetization of a salt, whose mean value is a function of temperature and can thus be used to infer the reservoir temperature. From statistical mecha ...