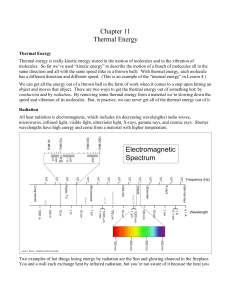
Affinity, Work, and Heat Introduction
... It is established in the text (Chapter 4) that the fundamental equation [4.8] is the equation for the tangent plane to the USV surface if the differential terms are of any arbitrary magnitude, but more importantly it is the equation for the USV surface itself. That means that integration of T dS − P ...
... It is established in the text (Chapter 4) that the fundamental equation [4.8] is the equation for the tangent plane to the USV surface if the differential terms are of any arbitrary magnitude, but more importantly it is the equation for the USV surface itself. That means that integration of T dS − P ...
Definite Integral and the Gibbs Paradox
... There are two ideal monatomic gases in an adiabatic container with volume V. The number of molecules are N1 and N 2 respectively. The temperature of the system in equilibrium is T. Find the equation of state, internal energy and entropy of the system, starting with canonical ensemble. Solution: Let ...
... There are two ideal monatomic gases in an adiabatic container with volume V. The number of molecules are N1 and N 2 respectively. The temperature of the system in equilibrium is T. Find the equation of state, internal energy and entropy of the system, starting with canonical ensemble. Solution: Let ...
2 - Kostic
... Work: The energy transfer associated with a force acting through a distance. A rising piston, a rotating shaft, and an electric wire crossing the system boundaries are all associated with work interactions Formal sign convention: Heat transfer to a system and work done by a system are positive; he ...
... Work: The energy transfer associated with a force acting through a distance. A rising piston, a rotating shaft, and an electric wire crossing the system boundaries are all associated with work interactions Formal sign convention: Heat transfer to a system and work done by a system are positive; he ...
Chapter 11 - Wolaver.org
... When you hold a cup of hot cocoa, your hand gets warm as the cardboard (the insulation) of the cup conducts heat from the liquid to your flesh. Whenever two materials are in contact, the material with the more energetic molecules transfers heat energy to the other material as touching molecules shak ...
... When you hold a cup of hot cocoa, your hand gets warm as the cardboard (the insulation) of the cup conducts heat from the liquid to your flesh. Whenever two materials are in contact, the material with the more energetic molecules transfers heat energy to the other material as touching molecules shak ...
Atmospheric Thermodynamics
... energy density – the number density of molecules n times kT . What does kT represent? It is not the internal energy density per molecule, which for translational energy alone is u̇trans = 32 kT > kT , and there is vibrational and rotational energy too, which makes the internal energy even bigger. Pe ...
... energy density – the number density of molecules n times kT . What does kT represent? It is not the internal energy density per molecule, which for translational energy alone is u̇trans = 32 kT > kT , and there is vibrational and rotational energy too, which makes the internal energy even bigger. Pe ...
Chapter 7 – Energy and Energy Balances
... The total internal energy, enthalpy, kinetic energy, and potential energy of a system are extensive properties. An extensive property depends on the total number of molecules present in the system and on the system’s total size. Often, it is more convenient to refer to the amount of a property per m ...
... The total internal energy, enthalpy, kinetic energy, and potential energy of a system are extensive properties. An extensive property depends on the total number of molecules present in the system and on the system’s total size. Often, it is more convenient to refer to the amount of a property per m ...
Download Pdf Article
... reproduced by all five equations of state. - equations SRK and PR can be used with the same temperature function with three parameters like the GEOS3C equation (eqs. 3-5). This fact leads to better values for vapor pressures but the values of saturated volumes can not be improved without a translati ...
... reproduced by all five equations of state. - equations SRK and PR can be used with the same temperature function with three parameters like the GEOS3C equation (eqs. 3-5). This fact leads to better values for vapor pressures but the values of saturated volumes can not be improved without a translati ...
PhysicsNotes QRECT Video Version With MetaNumber Feb 19 2013.pdf
... 2 Kinematics in One Dimension ....................................................................................................................... 10 2.1 Motion of an object in space - Define velocity & acceleration .............................................................. 10 2.2 Motion of o ...
... 2 Kinematics in One Dimension ....................................................................................................................... 10 2.1 Motion of an object in space - Define velocity & acceleration .............................................................. 10 2.2 Motion of o ...
Lecture Notes
... of compressive nature if it is heated, and vice versa. Another source for thermal stresses is thermal gradient within the body when a solid body is heated or cooled. It is because temperature distribution will depend on its size and shape. These thermal stresses may be established as a result of tem ...
... of compressive nature if it is heated, and vice versa. Another source for thermal stresses is thermal gradient within the body when a solid body is heated or cooled. It is because temperature distribution will depend on its size and shape. These thermal stresses may be established as a result of tem ...