The Power of Magnets
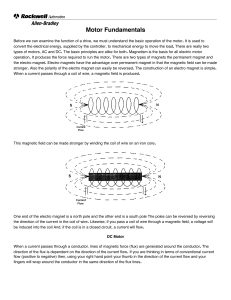
... magnets. Normally, the atoms in something like a lump of iron point in random directions and the individual magnetic fields tend to cancel each other out. However, the magnetic field produced by the wire wrapped around the core can force some of the atoms within the core to point in one direction. A ...
... magnets. Normally, the atoms in something like a lump of iron point in random directions and the individual magnetic fields tend to cancel each other out. However, the magnetic field produced by the wire wrapped around the core can force some of the atoms within the core to point in one direction. A ...
Transmission Electron Microscopy (no examples)
... • detects energy lost when exciting core electrons – analogous to X-ray absorption spectroscopy ...
... • detects energy lost when exciting core electrons – analogous to X-ray absorption spectroscopy ...
Physics 1001 - Introduction to Magnetism VO Magnets are all
... This is the first of many times that we’ll talk about the link between electrons in motion and magnetism. The fact is that moving charged particles, like electrons, produce magnetic fields. And it’s the spinning movement of electrons within the atom that is responsible for the atom’s magnetic proper ...
... This is the first of many times that we’ll talk about the link between electrons in motion and magnetism. The fact is that moving charged particles, like electrons, produce magnetic fields. And it’s the spinning movement of electrons within the atom that is responsible for the atom’s magnetic proper ...
Magnetic flux and Faraday`s Law
... (Michael Faraday conducted an experiment to prove this) At the moment one closes the switch ammeter deflect and returns immediately to zero. Again deflection at the moment opening the switch ...
... (Michael Faraday conducted an experiment to prove this) At the moment one closes the switch ammeter deflect and returns immediately to zero. Again deflection at the moment opening the switch ...
Magnets and the Magnetic field Part 1: The magnetic field of a
... regarding warm up time etc. and obtain the blue electron beam. This beam is a current, composed of electrons (negative charge carriers). In most uses of this apparatus, the coils are electrified to provide a magnetic field. For this exploration, the horseshoe magnet will provide the magnetic field, ...
... regarding warm up time etc. and obtain the blue electron beam. This beam is a current, composed of electrons (negative charge carriers). In most uses of this apparatus, the coils are electrified to provide a magnetic field. For this exploration, the horseshoe magnet will provide the magnetic field, ...
THE EFFECT OF MAGNETIC FIELD ON WATER HARDNESS
... (Fathi et al [7]). Magnetic treatment has been employed for more than a half century. The first commercial device was patented in Belgium in 1945 (Vemeiren [29]). Powerful electromagnets were used in hot water systems since the 1960s in the Soviet Union (Grutsch [10]). The application of magnetic tr ...
... (Fathi et al [7]). Magnetic treatment has been employed for more than a half century. The first commercial device was patented in Belgium in 1945 (Vemeiren [29]). Powerful electromagnets were used in hot water systems since the 1960s in the Soviet Union (Grutsch [10]). The application of magnetic tr ...
Spin-1/2 dynamics The intrinsic angular momentum of a spin
... We need to find the eigenvalues and eigenvectors. Consider the matrix without the factor of +h̄/2. Note that the trace defined to be the sum of the diagonal matrix elements. The trace is also the sum of the eigenvalues; denoting them by λ1 and λ2 we have λ1 + λ2 = 0. The determinant is easily calcul ...
... We need to find the eigenvalues and eigenvectors. Consider the matrix without the factor of +h̄/2. Note that the trace defined to be the sum of the diagonal matrix elements. The trace is also the sum of the eigenvalues; denoting them by λ1 and λ2 we have λ1 + λ2 = 0. The determinant is easily calcul ...
5G50.52 Energy Storage with Superconductors
... Finally, there is also a critical magnetic field strength, Bc , associated with a superconductor in a given state. The exact value of Bc depends on the temperature of, and current through, the superconductor. It also provides another means of classifying superconductors. In Type I superconductors th ...
... Finally, there is also a critical magnetic field strength, Bc , associated with a superconductor in a given state. The exact value of Bc depends on the temperature of, and current through, the superconductor. It also provides another means of classifying superconductors. In Type I superconductors th ...
Answers 6
... 4. Decide whether V is positive or negative by considering whether a positive charge would need to be pushed from the initial to the final position (Vfinal > Vinitial), or whether it would be pulled along by the field(Vfinal < Vinitial). Method 2 (Use the Principle of Superposition): 1. Break the g ...
... 4. Decide whether V is positive or negative by considering whether a positive charge would need to be pushed from the initial to the final position (Vfinal > Vinitial), or whether it would be pulled along by the field(Vfinal < Vinitial). Method 2 (Use the Principle of Superposition): 1. Break the g ...
A magnet is found to attract a steel ball. If the magnet is flipped
... A charged plastic rod is moved nearby a magnet that is free to rotate. What will happen? Nothing, if the magnet is perfectly vertical: the two sides of a magnet don’t have any net charge. If the charged rod is brought closer to one end or the other, it will attract that end due to the usual electric ...
... A charged plastic rod is moved nearby a magnet that is free to rotate. What will happen? Nothing, if the magnet is perfectly vertical: the two sides of a magnet don’t have any net charge. If the charged rod is brought closer to one end or the other, it will attract that end due to the usual electric ...
Magnetochemistry

Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.