The Core - WLWV Staff Blogs
... Outer Core • About 1790-3160 miles deep. • Liquid Core made of iron and nickel. • Currents in the outer core believed to cause the magnetic field. • Not under enough pressure to be solid. • Temperature believed to be 7200-9032 degrees Fahrenheit. ...
... Outer Core • About 1790-3160 miles deep. • Liquid Core made of iron and nickel. • Currents in the outer core believed to cause the magnetic field. • Not under enough pressure to be solid. • Temperature believed to be 7200-9032 degrees Fahrenheit. ...
B - Physics 420 UBC Physics Demonstrations
... • We measure magnetic field with a Gauss Meter • The field has direction so we will need to orient probe properly In this case parallel to the field lines (see Diagram) • If the field is negative field line is moving away from the probe Not Important we are only interested in |B| ...
... • We measure magnetic field with a Gauss Meter • The field has direction so we will need to orient probe properly In this case parallel to the field lines (see Diagram) • If the field is negative field line is moving away from the probe Not Important we are only interested in |B| ...
Magnetism and its uses
... Each spinning electron causes a magnetic field to form around it. Most materials have electrons that exist in pairs that spin in opposite directions thus canceling out the magnetic field. The atoms in materials such as iron, cobalt and nickel have unpaired electrons, so the electrons' magnetic field ...
... Each spinning electron causes a magnetic field to form around it. Most materials have electrons that exist in pairs that spin in opposite directions thus canceling out the magnetic field. The atoms in materials such as iron, cobalt and nickel have unpaired electrons, so the electrons' magnetic field ...
Organic Chemistry
... Organic Chemistry • The study of the compounds of carbon • Over 10 million compounds have been identified - about 1000 new ones are identified each day! ...
... Organic Chemistry • The study of the compounds of carbon • Over 10 million compounds have been identified - about 1000 new ones are identified each day! ...
Electric Circuits & Magnets
... about the magnetic field between two magnets? A. The south pole of one magnet is attracted to the south pole of the other magnet. B. The south pole of one magnet is attracted to the north pole of the other magnet. C. The north pole of one magnet is attracted to the north pole of the other magnet. D. ...
... about the magnetic field between two magnets? A. The south pole of one magnet is attracted to the south pole of the other magnet. B. The south pole of one magnet is attracted to the north pole of the other magnet. C. The north pole of one magnet is attracted to the north pole of the other magnet. D. ...
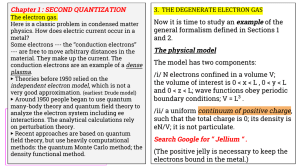
3. THE DEGENERATE ELECTRON GAS Chapter 1 : SECOND QUANTIZATION
... /i/ N electrons confined in a volume V; the volume of interest is 0 < x < L , 0 < y < L and 0 < z < L; wave functions obey periodic boundary conditions; V = L3 . /ii/ a uniform continuum of positive charge, such that the total charge is 0; its density is eN/V; it is not particulate. ...
... /i/ N electrons confined in a volume V; the volume of interest is 0 < x < L , 0 < y < L and 0 < z < L; wave functions obey periodic boundary conditions; V = L3 . /ii/ a uniform continuum of positive charge, such that the total charge is 0; its density is eN/V; it is not particulate. ...
Weekly Science Lesson Plans
... each group take turns using the magnets to pick up the different items inside their plastic bags. As a group they should decide what makes a material magnetic. ...
... each group take turns using the magnets to pick up the different items inside their plastic bags. As a group they should decide what makes a material magnetic. ...
Magnetochemistry

Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.