Atomic Structure
... • Found new type of particle in the atom that was electrically neutral – the neutron. • Determined mass of this particle. – Slightly larger than proton ...
... • Found new type of particle in the atom that was electrically neutral – the neutron. • Determined mass of this particle. – Slightly larger than proton ...
Atoms
... energy level model. The Lewis structure contains the element symbol with dots representing electrons. The only electrons shown are those on the outer energy level or valence electrons. The electrons are placed around the element symbol, one at a time, clockwise or counterclockwise, and then grouped ...
... energy level model. The Lewis structure contains the element symbol with dots representing electrons. The only electrons shown are those on the outer energy level or valence electrons. The electrons are placed around the element symbol, one at a time, clockwise or counterclockwise, and then grouped ...
4. bonding - New Hartford Central Schools
... Elements with more than one positive oxidation number (Transition Metals) (This is called the Stock System) When the oxidation number varies we us a Roman numeral in parentheses to indicate the charge. Roman number is used for the positive element only!!! ...
... Elements with more than one positive oxidation number (Transition Metals) (This is called the Stock System) When the oxidation number varies we us a Roman numeral in parentheses to indicate the charge. Roman number is used for the positive element only!!! ...
Test 1. 2nd prep. ques
... 2- The measuring unit of atomic radius which is used to measure the atomic size.(--picometer-) 3- Elements present in group zero.( noble gases ) 4- It is the series in which metals are arranged in a descending order according to their chemical activity . (--chemical activity series ) 5- Oxides of me ...
... 2- The measuring unit of atomic radius which is used to measure the atomic size.(--picometer-) 3- Elements present in group zero.( noble gases ) 4- It is the series in which metals are arranged in a descending order according to their chemical activity . (--chemical activity series ) 5- Oxides of me ...
SNC1D Periodic Table and Atomic Structure Package
... Unlike the naming of the elements, the system for determining the symbols follows a set of rules. In 1817, the system of chemical symbols that we use today was first proposed by the Swedish chemist Jons Jakob Berzelius (1779-1848). Eventually this system was accepted all around the world. It was a ...
... Unlike the naming of the elements, the system for determining the symbols follows a set of rules. In 1817, the system of chemical symbols that we use today was first proposed by the Swedish chemist Jons Jakob Berzelius (1779-1848). Eventually this system was accepted all around the world. It was a ...
Models of the Atom and Periodic Trends Worksheet
... rows 109. Atomic radius and metallic character increase as you go from bottom to top and from left to right across the periodic table. 110. The periodic table can be broken down into blocks where a certain sublevel is being filled. 111. Valence electrons are the outermost electrons and are involved ...
... rows 109. Atomic radius and metallic character increase as you go from bottom to top and from left to right across the periodic table. 110. The periodic table can be broken down into blocks where a certain sublevel is being filled. 111. Valence electrons are the outermost electrons and are involved ...
THE STRUCTURE OF THE ATOM
... • Dalton’s atomic theory was a huge step towards our current atomic model of matter but like most theories as technology and knowledge develops the models used also develop and change. • As it turns out there were a couple of items in Dalton’s theory that aren’t quite correct. ...
... • Dalton’s atomic theory was a huge step towards our current atomic model of matter but like most theories as technology and knowledge develops the models used also develop and change. • As it turns out there were a couple of items in Dalton’s theory that aren’t quite correct. ...
Periodic Table
... The zigzag line on the periodic table helps you to recognize which elements are metals, nonmetals and ...
... The zigzag line on the periodic table helps you to recognize which elements are metals, nonmetals and ...
Assignment
... of the Atom – Quantum Mechanics Bohr’s model worked well in explaining the behaviour of simple atoms such as hydrogen, that contained few electrons, but it did not explain the more complex atoms. ...
... of the Atom – Quantum Mechanics Bohr’s model worked well in explaining the behaviour of simple atoms such as hydrogen, that contained few electrons, but it did not explain the more complex atoms. ...
Chapter 7 Periodic Properties of the Elements
... the same conclusion about how elements should be grouped in the periodic table. •Henry Moseley (1913) developed the concept of atomic numbers (the number of protons in the nucleus of an atom) ...
... the same conclusion about how elements should be grouped in the periodic table. •Henry Moseley (1913) developed the concept of atomic numbers (the number of protons in the nucleus of an atom) ...
File
... 1) What was Democritus’ Theory? If you continued to cut something into smaller pieces, you eventually reach a point you can divide it any more….called it atomos or an atom. 2) Who was Dalton? English school teacher. What are the three parts to his famous theory?1. All matter composed of small partic ...
... 1) What was Democritus’ Theory? If you continued to cut something into smaller pieces, you eventually reach a point you can divide it any more….called it atomos or an atom. 2) Who was Dalton? English school teacher. What are the three parts to his famous theory?1. All matter composed of small partic ...
File
... was made of small bits by looking at sand on the beach. He pounded up materials in his pestle and mortar until he had reduced them to smaller and smaller particles which he called ...
... was made of small bits by looking at sand on the beach. He pounded up materials in his pestle and mortar until he had reduced them to smaller and smaller particles which he called ...
Atoms and the Periodic Table
... • Explain why some atoms gain or lose electrons to form ions. • Determine how many protons, neutrons, and electrons an atom has, given its’ symbol, atomic number, and mass number • Describe how the abundance of isotopes affects an element’s average atomic mass. ...
... • Explain why some atoms gain or lose electrons to form ions. • Determine how many protons, neutrons, and electrons an atom has, given its’ symbol, atomic number, and mass number • Describe how the abundance of isotopes affects an element’s average atomic mass. ...
12.1 Atoms
... • Isotopes are atoms of the same element that have different number of neutrons. • If the number of neutrons change, the mass of the atom changes • mass # = protons + neutrons ...
... • Isotopes are atoms of the same element that have different number of neutrons. • If the number of neutrons change, the mass of the atom changes • mass # = protons + neutrons ...
atomic number on the periodic table
... History of the Periodic Table • Dmitri Mendeleev, a Russian scientist born in Siberia in 1834, is known as the father of the periodic table of the elements • The periodic table is designed to help you predict chemical and physical properties of elements ...
... History of the Periodic Table • Dmitri Mendeleev, a Russian scientist born in Siberia in 1834, is known as the father of the periodic table of the elements • The periodic table is designed to help you predict chemical and physical properties of elements ...
Name Class Block ______ Directions: Read the following article
... Dalton correctly suggested that each element’s atoms are identical. He based his theory on the way elements combined into compounds. Dalton could not look inside an atom and did not fully understand atomic structure. Despite this, his ideas inspired more than a century’s worth of research and scien ...
... Dalton correctly suggested that each element’s atoms are identical. He based his theory on the way elements combined into compounds. Dalton could not look inside an atom and did not fully understand atomic structure. Despite this, his ideas inspired more than a century’s worth of research and scien ...
The Periodic Table of Elements Mendeleev
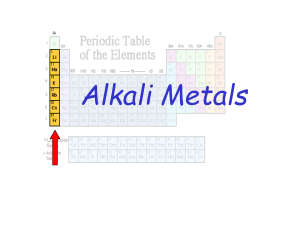
... • Vertical columns of elements in the periodic table are called groups. • Elements in the same group or family have similar chemical properties. • Main Group (Representative) Elements are those in the first two groups and the last six groups in the periodic table. These groups are designated IA - VI ...
... • Vertical columns of elements in the periodic table are called groups. • Elements in the same group or family have similar chemical properties. • Main Group (Representative) Elements are those in the first two groups and the last six groups in the periodic table. These groups are designated IA - VI ...
Ch 17 Notes
... atoms of the same kind make up an element atoms w/ same # of protons belong to same element atoms w/ diff. # of protons are different elements 115 elements known today 90 elements occur in nature, rest made in laboratory with high-energy accelerators and nuclear reactors [ these are too unstable (ra ...
... atoms of the same kind make up an element atoms w/ same # of protons belong to same element atoms w/ diff. # of protons are different elements 115 elements known today 90 elements occur in nature, rest made in laboratory with high-energy accelerators and nuclear reactors [ these are too unstable (ra ...
ChemCh4and6of2011
... Thomson believed that the electrons were like plums embedded in a positively charged “pudding,” thus it was called the “plum pudding” model. Based on the following facts: (1) atoms contain small, negatively charged particles called electrons and (2) the atoms of the element behave as if they have no ...
... Thomson believed that the electrons were like plums embedded in a positively charged “pudding,” thus it was called the “plum pudding” model. Based on the following facts: (1) atoms contain small, negatively charged particles called electrons and (2) the atoms of the element behave as if they have no ...
Atomic Structure
... C. Milliken’s experiments allow for 2 inferences (conclusions based upon evidence and reasoning) to be made: 1. Because atoms, in the natural state, are electrically neutral, they must also contain an equal amount of positively charged particles. 2. Because electrons have so little mass, atoms must ...
... C. Milliken’s experiments allow for 2 inferences (conclusions based upon evidence and reasoning) to be made: 1. Because atoms, in the natural state, are electrically neutral, they must also contain an equal amount of positively charged particles. 2. Because electrons have so little mass, atoms must ...
Section 15.1
... The mass of individual atoms is so small that the numbers are difficult to work with. To make calculations easier, scientists use the atomic mass unit (amu). The atomic mass of any element is the average mass (in amu) of an atom of each element. ...
... The mass of individual atoms is so small that the numbers are difficult to work with. To make calculations easier, scientists use the atomic mass unit (amu). The atomic mass of any element is the average mass (in amu) of an atom of each element. ...