4.1 Defining the Atom - Miami Beach Senior High School
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
TEKS Presentation Properties of Matter
... retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
... retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
4.1 Defining the Atom - Miami Beach Senior High School
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
4.1 Defining the Atom - Pittsfield High School
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
CHEM12 C04 L1 LO File
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
2.1 Atomic Theory of Matter
... The total mass of substances present at the end of a chemical process is the same as the mass of substances present before the ...
... The total mass of substances present at the end of a chemical process is the same as the mass of substances present before the ...
Chemistry - Textbooks Online
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
Плеханов В
... Isotope effects reason is nuclei mass difference. The formula for -factor calculation from oscillations kinetic energy of isotope-substituting atom K [12]. Isotope mixture rectification. The substance transition between condensed and gaseous phase related to intermolecular bonds destruction has iso ...
... Isotope effects reason is nuclei mass difference. The formula for -factor calculation from oscillations kinetic energy of isotope-substituting atom K [12]. Isotope mixture rectification. The substance transition between condensed and gaseous phase related to intermolecular bonds destruction has iso ...
No Slide Title - MDC Faculty Home Pages
... Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition ...
... Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition ...
James Moir as Inorganic Chemist
... He supplied a long table with proposed compositions of the nuclei of the elements, of which the first 15 elements are shown in Table 1. Generally, agreement was very good, for that time. He suggested that the new scheme brought out closer relationships between such groups as the alkali metals and th ...
... He supplied a long table with proposed compositions of the nuclei of the elements, of which the first 15 elements are shown in Table 1. Generally, agreement was very good, for that time. He suggested that the new scheme brought out closer relationships between such groups as the alkali metals and th ...
Final Exam Review Notes
... Postulate from John Dalton’s Model of the Atom 1. All matter is composed of indivisible atoms. 2. An element is composed of only one type of atom – All atoms of one type of element always behave the same way. – Atoms of different elements do not behave the same way. 3. Two or more elements combine t ...
... Postulate from John Dalton’s Model of the Atom 1. All matter is composed of indivisible atoms. 2. An element is composed of only one type of atom – All atoms of one type of element always behave the same way. – Atoms of different elements do not behave the same way. 3. Two or more elements combine t ...
4.1 Defining the Atom
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
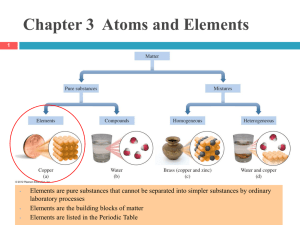
Chapter 2 Atoms and Elements If You Cut a Piece of Graphite • If you
... • Main-group elements are in columns labeled with a number and the letter A (1A–8A or groups 1, 2, and 13–18). ...
... • Main-group elements are in columns labeled with a number and the letter A (1A–8A or groups 1, 2, and 13–18). ...
Chapter 2: Atoms, Ions, and the Periodic Table
... D) When a metal reacts with oxygen, the oxygen content of the products is fixed at one or two values. E) When water is broken down into its elements by electrolysis, elemental oxygen and hydrogen are formed in an 8 to 1 mass ratio. Ans: C 8. Which of the following is not part of Dalton's atomic theo ...
... D) When a metal reacts with oxygen, the oxygen content of the products is fixed at one or two values. E) When water is broken down into its elements by electrolysis, elemental oxygen and hydrogen are formed in an 8 to 1 mass ratio. Ans: C 8. Which of the following is not part of Dalton's atomic theo ...
Chapter 2: Atoms, Ions, and the Periodic Table
... D) When a metal reacts with oxygen, the oxygen content of the products is fixed at one or two values. E) When water is broken down into its elements by electrolysis, elemental oxygen and hydrogen are formed in an 8 to 1 mass ratio. Ans: C 8. Which of the following is not part of Dalton's atomic theo ...
... D) When a metal reacts with oxygen, the oxygen content of the products is fixed at one or two values. E) When water is broken down into its elements by electrolysis, elemental oxygen and hydrogen are formed in an 8 to 1 mass ratio. Ans: C 8. Which of the following is not part of Dalton's atomic theo ...
Preview Sample 1
... LO: 02.01.05 Explain how a single element may exist in more than one form, called isotopes, and how certain isotopes have importance in human medicine. Section: 02.01 Atoms Topic: Chemistry ...
... LO: 02.01.05 Explain how a single element may exist in more than one form, called isotopes, and how certain isotopes have importance in human medicine. Section: 02.01 Atoms Topic: Chemistry ...
File
... For electrons, protons and neutrons – write down the charge on each particle, the mass of each particle, and where they are found in the atom. Electron = neg. charge found outside nucleus, almost zero mass Protons = pos. charge, in nucleus, 1 amu Neutron = no charge, in nucleus, 1 amu ...
... For electrons, protons and neutrons – write down the charge on each particle, the mass of each particle, and where they are found in the atom. Electron = neg. charge found outside nucleus, almost zero mass Protons = pos. charge, in nucleus, 1 amu Neutron = no charge, in nucleus, 1 amu ...
intro to atomic theory ppt
... Thomson’s Model of the Atom When the current was turned on, the disks became charged, and a glowing beam appeared in the tube. • Thomson hypothesized that the beam was a stream of charged particles that interacted with the air in the tube and caused the air to glow. • Thomson observed that the beam ...
... Thomson’s Model of the Atom When the current was turned on, the disks became charged, and a glowing beam appeared in the tube. • Thomson hypothesized that the beam was a stream of charged particles that interacted with the air in the tube and caused the air to glow. • Thomson observed that the beam ...