Investigations Concerning the Laser Ignition of Energetic
... PETN, or LA) are still in use and commercially produced on a large scale. Thus, RDX for example still represents the world's number one military secondary explosive. During world war second, development of so-called polymer-bonded explosives (PBX) started to reduce the sensitivity and simplifying th ...
... PETN, or LA) are still in use and commercially produced on a large scale. Thus, RDX for example still represents the world's number one military secondary explosive. During world war second, development of so-called polymer-bonded explosives (PBX) started to reduce the sensitivity and simplifying th ...
Lanthanides and actinides. Annual survey of their organometallic
... whose structure reveals that the two anionic [(C5Me,) (C2B,H,,)ScH] fragments are held together by reciprocal B-H dative bonds from the dicarbollide ligand to the electron deficient scandium. The potential of pentamethylcyclopentadienyl-dicarbollide derivatives of scandium to serve as efficient a-ol ...
... whose structure reveals that the two anionic [(C5Me,) (C2B,H,,)ScH] fragments are held together by reciprocal B-H dative bonds from the dicarbollide ligand to the electron deficient scandium. The potential of pentamethylcyclopentadienyl-dicarbollide derivatives of scandium to serve as efficient a-ol ...
Noncovalent interactions of molecules with single walled carbon
... was suggested that an energy correction of 1–2% for curvature could be expected, based on similar corrections for curvatureinduced changes in electronic structure,33 though p orbital misalignment may introduce larger differences in binding energy.31 Often, rather than using a discrete potential, a c ...
... was suggested that an energy correction of 1–2% for curvature could be expected, based on similar corrections for curvatureinduced changes in electronic structure,33 though p orbital misalignment may introduce larger differences in binding energy.31 Often, rather than using a discrete potential, a c ...
Molecular Encapsulation - Colin Nuckolls
... La Jolla, California. He has been awarded postgraduate fellowships by the Natural Sciences and Engineering Research Council of Canada and the Skaggs Institute for Chemical Biology. His research focuses on the rational design of novel emergent properties through supramolecular chemistry. Stephen Crai ...
... La Jolla, California. He has been awarded postgraduate fellowships by the Natural Sciences and Engineering Research Council of Canada and the Skaggs Institute for Chemical Biology. His research focuses on the rational design of novel emergent properties through supramolecular chemistry. Stephen Crai ...
TRIARYLBORON COMPOUNDS AND THEIR PLATINUM(II) COMPLEXES: PHOTOPHYSICAL PROPERTIES AND APPLICATIONS IN OPTOELECTRONICS
... emphasis on organoboron- and organoplatinum-containing compounds. The preparation of a nonconjugated two-chromophore emissive material is described, containing both organoplatinum and organoboron units. This material exhibits simultaneous fluorescent and phosphorescent emission at ambient temperatur ...
... emphasis on organoboron- and organoplatinum-containing compounds. The preparation of a nonconjugated two-chromophore emissive material is described, containing both organoplatinum and organoboron units. This material exhibits simultaneous fluorescent and phosphorescent emission at ambient temperatur ...
Benzene - Serrata
... of 140 picometres (pm). The C–C bond lengths are greater than a double bond (135pm) but shorter than a single bond (147pm). This intermediate distance is explained by electron delocalization: the electrons for C–C bonding are distributed equally between each of the six carbon atoms. The molecule is ...
... of 140 picometres (pm). The C–C bond lengths are greater than a double bond (135pm) but shorter than a single bond (147pm). This intermediate distance is explained by electron delocalization: the electrons for C–C bonding are distributed equally between each of the six carbon atoms. The molecule is ...
Two-electron Quenching of Dinuclear Ruthenium(II)
... The excited state decays involve several processes (Fig 1.4). By irradiating Ru complexes, for example [Ru(bpy)3]2+, at the appropriate wavelength, a lowest photoexcited singlet metal-toligand charge transfer state (1MLCT) is produced, 1[Ru(bpy)3]2+* , which then undergoes intersystem crossing (kis ...
... The excited state decays involve several processes (Fig 1.4). By irradiating Ru complexes, for example [Ru(bpy)3]2+, at the appropriate wavelength, a lowest photoexcited singlet metal-toligand charge transfer state (1MLCT) is produced, 1[Ru(bpy)3]2+* , which then undergoes intersystem crossing (kis ...
$doc.title
... Thiophenes are well known for their occurrence in fossil fuels o2 and the coordination chemistry of these compounds has received recent attention because of its relevance to the metal-catalyzed hydrodesulfurization of the fossil fuels 63 . A mechanism for the hydrodesulfurization was proposed by Ang ...
... Thiophenes are well known for their occurrence in fossil fuels o2 and the coordination chemistry of these compounds has received recent attention because of its relevance to the metal-catalyzed hydrodesulfurization of the fossil fuels 63 . A mechanism for the hydrodesulfurization was proposed by Ang ...
Structural Identification of Doped Silicon Clusters
... matter of fact, both experiment and theory have been facing difficulties and challenges to investigate the structures of clusters. Computationally, many energetically low-lying isomers may exist for a given cluster size, with small energy differences that are within the accuracy of contemporary metho ...
... matter of fact, both experiment and theory have been facing difficulties and challenges to investigate the structures of clusters. Computationally, many energetically low-lying isomers may exist for a given cluster size, with small energy differences that are within the accuracy of contemporary metho ...
Extremely Bulky Amido d-Block Metal(II) Halide Complexes
... to the metal centre, yielding a square planar geometry, similar to 2-06. This structural change has come about as a result of a fluorine atom in the para position of the terphenyl ligand central ring in 2-19, or the bridging fluorides in 2-20. In both cases, the highly electronegative fluorine atoms ...
... to the metal centre, yielding a square planar geometry, similar to 2-06. This structural change has come about as a result of a fluorine atom in the para position of the terphenyl ligand central ring in 2-19, or the bridging fluorides in 2-20. In both cases, the highly electronegative fluorine atoms ...
Cadmiumcomplexes bearing N^E^O (E = S, Se
... molecular solid-state structures, except for 11, the structure of which could not be ascertained. ...
... molecular solid-state structures, except for 11, the structure of which could not be ascertained. ...
$doc.title
... to be 8 mol% Pd(PPh3)4, 32 mol% PPh3, 1.5 eq. Ag2O and 1.5 eq. K2CO3 in DME at 85 °C for 24 h. This gave the desired coupling product in 64 % yield with 99.5 % retention of stereoch ...
... to be 8 mol% Pd(PPh3)4, 32 mol% PPh3, 1.5 eq. Ag2O and 1.5 eq. K2CO3 in DME at 85 °C for 24 h. This gave the desired coupling product in 64 % yield with 99.5 % retention of stereoch ...
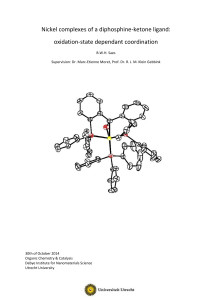
Nickel complexes of a diphosphine-ketone ligand: oxidation
... activation reaction type by the metal centred catalyst. Different H-H σ-bond activation types have been established[29,30], as can be seen in Figure 2. In the homolytic pathway ((A) in Figure 2), the two hydrogen atoms are both oxidatively added to the metal centre, yielding two chemically near-equi ...
... activation reaction type by the metal centred catalyst. Different H-H σ-bond activation types have been established[29,30], as can be seen in Figure 2. In the homolytic pathway ((A) in Figure 2), the two hydrogen atoms are both oxidatively added to the metal centre, yielding two chemically near-equi ...
Dihalogeno(diphosphane)metal(II) complexes (metal Co, Ni, Pd) as
... temperature factors. The hydrogen atoms were placed at calculated positions, using appropriate riding models (HFIX 43 for aromatic CH, HFIX 33 for CH3, HFIX 23 for CH2, HFIX 153 for CH and BH in the carborane, HFIX 83 for OH) and isotropic temperature factors of U(H) = 1.2 Ueq(BH, CH and CH2) or U(H ...
... temperature factors. The hydrogen atoms were placed at calculated positions, using appropriate riding models (HFIX 43 for aromatic CH, HFIX 33 for CH3, HFIX 23 for CH2, HFIX 153 for CH and BH in the carborane, HFIX 83 for OH) and isotropic temperature factors of U(H) = 1.2 Ueq(BH, CH and CH2) or U(H ...
Small Reactive Sulfur-Nitrogen Compounds and Their Transition
... of stable salts such as SN+AsF,, SN;SbF; and K2SN2. 2. The (thermal or photochemical) generation of highly reactive molecules from suitable precursors has allowed the spectroscopic characterization of transients such as SN and SN,, either in the gas phase at low pressures or in a low-temperature mat ...
... of stable salts such as SN+AsF,, SN;SbF; and K2SN2. 2. The (thermal or photochemical) generation of highly reactive molecules from suitable precursors has allowed the spectroscopic characterization of transients such as SN and SN,, either in the gas phase at low pressures or in a low-temperature mat ...
Chapter 16 Solubility Equilibrium
... • Consider the following equilibrium: Mg(OH)2(s) ⇌ Mg2+(aq) + 2 OH-(aq); • Increasing the pH means increasing [OH-] and equilibrium will shift to the left, causing some of Mg(OH)2 to precipitate out. • If the pH is lowered, [OH-] decreases and equilibrium shifts to the right, causing solid Mg(OH)2 t ...
... • Consider the following equilibrium: Mg(OH)2(s) ⇌ Mg2+(aq) + 2 OH-(aq); • Increasing the pH means increasing [OH-] and equilibrium will shift to the left, causing some of Mg(OH)2 to precipitate out. • If the pH is lowered, [OH-] decreases and equilibrium shifts to the right, causing solid Mg(OH)2 t ...
g now! - Repositori UJI
... confinement effects. Thus, the study of this nanosized semiconductor is of considerable importance and great efforts have been focused on their synthesis and physical properties.6 As one of the most important semiconductors, ZnS has been known for a long time as a versatile and excellent phosphor ho ...
... confinement effects. Thus, the study of this nanosized semiconductor is of considerable importance and great efforts have been focused on their synthesis and physical properties.6 As one of the most important semiconductors, ZnS has been known for a long time as a versatile and excellent phosphor ho ...
Full-Text PDF
... Anderson and Merckling published their observation of methine exchange by scission-migration of carbon–carbon double bonds in titanium-catalyzed polymerization of norbornene [1,2]. One decade later, Banks and Bailey reported a new comproportionation reaction converting dissymmetric alkenes to symmet ...
... Anderson and Merckling published their observation of methine exchange by scission-migration of carbon–carbon double bonds in titanium-catalyzed polymerization of norbornene [1,2]. One decade later, Banks and Bailey reported a new comproportionation reaction converting dissymmetric alkenes to symmet ...
A computational study of CH4 storage in porous
... MOFs is by introduction of open-metal sites into their structure.20–22 Most open-metal sites have a signicantly stronger interaction energy with CH4 than other adsorption sites on the pore surface of a MOF, and therefore, MOFs containing high concentrations of open-metal sites are expected to have ...
... MOFs is by introduction of open-metal sites into their structure.20–22 Most open-metal sites have a signicantly stronger interaction energy with CH4 than other adsorption sites on the pore surface of a MOF, and therefore, MOFs containing high concentrations of open-metal sites are expected to have ...
Coordination Chemistry Reviews Prediction of molecular properties
... 1. Introductory remarks The present volume is devoted to the use of density functional theory (DFT) in (bio)-inorganic chemistry. In fact, DFT has enjoyed an enormous popularity in this field over the past two decades (for reviews see [1–16]) and has found many users that range from hardcore theoreti ...
... 1. Introductory remarks The present volume is devoted to the use of density functional theory (DFT) in (bio)-inorganic chemistry. In fact, DFT has enjoyed an enormous popularity in this field over the past two decades (for reviews see [1–16]) and has found many users that range from hardcore theoreti ...
Notes 6 - Macdonald Research Group
... N-heterocyclic carbenes (NHC) have become one of the most useful and investigated classes of ligands since their discovery. NHCs are very basic and they are very strong nucleophiles. This makes them excellent donors that form stronger bonds to transition metals than ligands such as phosphines. The a ...
... N-heterocyclic carbenes (NHC) have become one of the most useful and investigated classes of ligands since their discovery. NHCs are very basic and they are very strong nucleophiles. This makes them excellent donors that form stronger bonds to transition metals than ligands such as phosphines. The a ...
Jahn–Teller effect
-3D-balls.png?width=300)
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that is associated with certain electron configurations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory, that orbital nonlinear spatially degenerate molecules cannot be stable. The Jahn–Teller theorem essentially states that any nonlinear molecule with a spatially degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the species. For a description of another type of geometrical distortion that occurs in crystals with substitutional impurities see article off-center ions.